High Temperature Materials
Abstract
This comprehensive review examines high temperature materials, with a particular focus on superalloys and their mechanical properties at elevated temperatures. The article discusses three main categories of superalloys - iron-based, nickel-based, and cobalt-based - analyzing their strength characteristics, temperature limitations, and microstructural features. Special attention is given to their stress-rupture properties and strengthening mechanisms through various phases. The study compares different metallic alloys' performance at high temperatures and explores the critical role of corrosion resistance in material selection for high-temperature applications.
Introduction to High Temperature Materials
The field of high temperature materials encompasses a diverse range of materials engineered for elevated temperature applications. When operating at high temperatures, a material's performance is primarily evaluated through its tensile strength, stress rupture life, and fatigue life characteristics. Additionally, corrosion resistance becomes a crucial factor, as degradation processes can significantly impact material strength at elevated temperatures.
Temperature Limitations and Material Performance
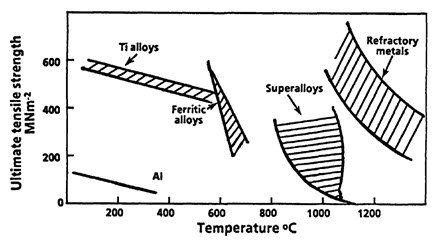
Figure 1: Tensile strengths of a number of alloys as a function of temperature to indicate temperature ranges over which different alloy systems may be used.
Different materials exhibit varying maximum use temperatures and strength characteristics. As illustrated in Figure 1, refractory metals demonstrate superior strength at temperatures reaching 1,200°C, though their oxidation resistance remains a limitation. Nickel and cobalt-based superalloys maintain robust strength properties up to 1,000°C while offering acceptable oxidation resistance.
In the intermediate temperature range, titanium alloys and certain steels perform effectively up to 600-700°C. Aluminum alloys, while limited in high-temperature applications, can achieve improved performance through fiber reinforcement, reaching flexural strengths of 300-400 MPa at 300°C.
Superalloy Categories and Characteristics
The field of superalloys encompasses three primary categories: iron (iron-nickel)-based, nickel-based, and cobalt-based alloys. These advanced materials were specifically engineered to deliver exceptional strength and corrosion resistance in high-temperature environments. A distinguishing characteristic of these alloys is their ability to maintain their mechanical properties even after prolonged exposure to elevated temperatures.
What sets superalloys apart is their unique combination of properties: high-temperature strength, excellent operating temperature ranges, good low-temperature ductility, and superior surface stability. This versatility makes them invaluable in demanding applications where multiple performance criteria must be met simultaneously.
While conventional metals are typically evaluated based on short-term properties like yield strength and ultimate tensile strength at moderate temperatures, superalloys require a different assessment approach. Their performance is best evaluated at temperatures reaching or exceeding 50% of their absolute melting point, as these materials are designed for sustained high-temperature service conditions.
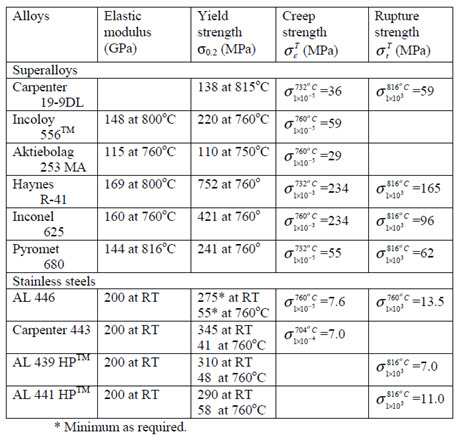
Table 1. High temperature mechanical properties of super-alloy and stainless steels.
Stress-Rupture Performance
Stress-rupture testing, also known as creep-rupture testing, serves as a critical evaluation method for superalloys, providing essential data about their long-term strength capabilities and potential applications. The steady-state creep rate derived from these tests is particularly significant for engineering design considerations.
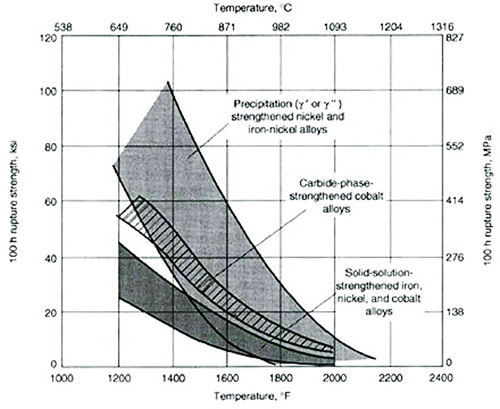
Figure 2: Creep-rupture strengths of superalloys
Analysis of the creep-rupture test results, as illustrated in Figure 2, demonstrates that iron-nickel and nickel-based superalloys exhibit superior stress-rupture strengths compared to their carbide-phase strengthened cobalt counterparts and solid-solution-strengthened variants. This enhanced performance can be attributed to several complex microstructural features:
Microstructural Strengthening Mechanisms
- Primary Matrix Structure (γ Matrix): The foundation of these alloys is a continuous face-centered cubic (FCC) nickel-based phase, incorporating high concentrations of solid-solution elements including cobalt, iron, chromium, molybdenum, and tungsten.
- Strengthening Precipitates (γ' Phases): These crucial strengthening elements form from aluminum and titanium combinations, creating Ni3(Al,Ti) compounds. These precipitates maintain coherency with the austenitic γ matrix, with both phases exhibiting cubic structures but different lattice parameters. This lattice mismatch generates coherency strains that effectively impede dislocation movement. The precipitates typically appear as spheres or cuboids in a bimodal distribution pattern, serving as the primary strengthening mechanism at elevated temperatures.
- Grain Boundary Features: Heat treatments and service exposure produce γ' films along grain boundaries, enhancing creep-rupture properties. Under stress conditions, these precipitates can form elongated structures known as γ' rafts, whose effectiveness in strengthening depends on the γ/γ' misfit characteristics.
- Secondary Strengthening Phases (γ'' Phases): In iron-containing systems, nickel and niobium combine to form a body-centered tetragonal (BCT) phase (Ni3Nb) that maintains coherency with the γ matrix. While this phase provides significant strength at lower temperatures through large mismatch strains, it becomes unstable at higher temperatures, potentially transforming into a less beneficial δ phase (orthorhombic Ni3Nb intermetallic).
- Carbide Formations: Carbon interactions with elements such as titanium, tantalum, hafnium, and niobium produce various metal carbides (MC, M23C6, M6C, and M7C3). While these carbides offer some direct strengthening through dispersion hardening, their primary benefit in polycrystalline materials comes from stabilizing grain boundaries against excessive shear, provided the carbon content remains below solubility limits.
This microstructural complexity explains why joining operations involving γ'' forming alloys typically require carefully controlled heat treatments to prevent the formation of detrimental intermetallic phases. Additionally, the role of carbon in strengthening explains its typical exclusion from single crystal alloy compositions.
Access Creep Properties of Thousands of Materials Now!
Total Materia Horizon includes the largest database of creep data such as yield stress and creep rupture strength at different temperatures, for thousands of metallic alloys and polymers.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.