The Oxygen Steelmaking Process
Abstract
The oxygen steelmaking process encompasses methods that utilize gaseous oxygen as the primary agent for autothermic heat generation through oxidation of dissolved impurities including carbon, silicon, manganese, and phosphorus. This process includes various techniques such as top blowing, bottom blowing, and combined blowing methods. The Basic Oxygen Furnace (BOF) process transforms pig iron containing approximately 4.2% carbon into refined steel through controlled oxidation reactions. Key refining reactions involve carbon removal, silicon oxidation, manganese control, phosphorus partitioning, and sulfur removal. The process efficiency depends on optimal slag basicity, temperature control, and oxygen flow management, making it the dominant steelmaking technology worldwide.
Introduction to Oxygen Steelmaking Technology
The oxygen steelmaking process represents a revolutionary approach to steel production, where gaseous oxygen serves as the primary oxidizing agent for autothermic heat generation. This heat results from the oxidation of dissolved impurities such as carbon, silicon, manganese, and phosphorus, along with limited iron oxidation. The steel industry has developed several oxygen steelmaking variants, including top blowing, bottom blowing, and combined blowing processes, each offering unique advantages for specific production requirements.
Modern steelmaking focuses on the partial oxidation of carbon, silicon, phosphorus, and manganese present in pig iron while simultaneously reducing sulfur levels. The Basic Oxygen Furnace (BOF) process, also known as the LD process, typically uses blast furnace hot metal with optimal composition: carbon at 4.2%, silicon maximum 0.8%, manganese maximum 0.8%, sulfur maximum 0.05%, and phosphorus maximum 0.15%. These solute elements become diluted through scrap addition, which constitutes 20-30% of the metallic charge.
Fundamental Refining Reactions in Steel Production
Oxygen Distribution and Iron Oxidation
In the LD basic oxygen steelmaking process, oxygen supply for refining reactions occurs in gaseous form, with both metal and slag undergoing initial oxidation. The primary reactions include:
½O2(g) ↔ [O] .....(1)
Fe + [O] ↔ (FeO) .....(2)
2(FeO) + ½O2(g) ↔ (Fe2O3) .....(3)
The actual oxygen distribution between slag and metal depends on multiple variables, including lance height and oxygen flow rate, making precise determination challenging during operation.
Carbon Removal: The Primary Steelmaking Reaction
Carbon removal represents the most critical refining reaction in oxygen steelmaking:
[C] + [O] ↔ CO2 .....(4)
[C] + (FeO) ↔ CO2 + Fe .....(5)
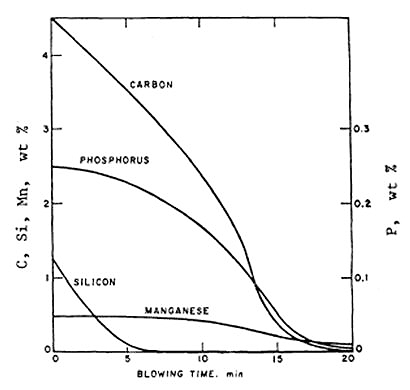
Figure 1: The changes of bath composition during the blow in a basic oxygen steelmaking converter (idealized)
Figure 1 illustrates the idealized changes in bath composition throughout the blowing process. The thermodynamic data for these reactions are well-established, enabling accurate calculation of equilibrium carbon and oxygen contents across all steelmaking temperatures and pressures.
Carbon oxidation during the oxygen converter process holds paramount importance because this reaction increases temperature and evolves substantial quantities of CO and CO₂ gases. These gases create vigorous agitation of metal and slag while removing hydrogen, nitrogen, and non-metallic inclusions from the metal. The combination of oxygen pressure and extensive gas evolution transforms the liquid bath into an intimate mixture of slag, metal, and gas bubbles with enormous contact surface area. This phenomenon makes carbon oxidation self-accelerating and achieves exceptionally high reaction rates.
Silicon Oxidation and Removal
Silicon removal typically completes early in the blowing process, following thermodynamic predictions. The governing reactions are:
[Si] + 2[O] ↔ (SiO₂) .....(6)
[Si] + 2(FeO) ↔ (SiO₂) + 2Fe .....(7)
The early completion of silicon oxidation results from its high affinity for oxygen and the favorable thermodynamic conditions present during initial blowing stages.
Manganese Behavior During Oxygen Steelmaking
Manganese removal follows similar oxidation patterns:
[Mn] + [O] ↔ (MnO) .....(8)
[Mn] + (FeO) ↔ (MnO) + Fe .....(9)
Initially, bath manganese levels decrease due to oxidation, followed by slight reversion and subsequent decline. These manganese content variations result from combined effects of rising temperature and variable slag composition on manganese and ferrous oxide activities, indicating near-equilibrium conditions. Research demonstrates that manganese content reaches 82% of equilibrium value with lump lime and 85% with powdered lime injection.
During the middle blowing phase, slag FeO levels fall due to decarburization and lime fluxing dilution. However, toward blow completion, FeO increases as carbon removal intensity decreases and dilution affects manganese oxide activity, causing manganese transfer from bath to slag. Temperature elevation can minimize manganese losses to some extent.
Phosphorus Partitioning and Control
Thermodynamic Principles of Phosphorus Distribution
Phosphorus partitioning between slag and metal demonstrates high sensitivity to process conditions, making kinetic modeling based on simple assumptions challenging. Healy's comprehensive review concluded that phosphorus thermodynamic behavior follows a modified ionic theory originally proposed by Flood and Grjotheim. The slag-metal reaction appears in ionic form:
2[P] + 5[O] + 3(O²⁻) ↔ 2(PO₄³⁻) .....(10)
Healy developed equilibrium distribution equations for specific CaO-SiO₂-FeO system concentration ranges:
log (%P)/[P] = 22 350/T + 7 log%CaO + 2.5 log Fet – 24.0 .....(11)
log (%P)/[P] = 22 350/T + 0.08 log%CaO + 2.5 log Fet – 16.0 .....(12)
Equation (11) applies to slags containing over 24% CaO, while equation (12) remains valid from zero %CaO to saturation levels.
Practical Phosphorus Removal Challenges
In practice, phosphorus partition ratios deviate significantly from equilibrium calculations with carbon-free iron because decarburization influences the slag-metal system's oxygen potential. Limited correlation with bath carbon content has been reported, though some researchers suggest extensive dephosphorization remains possible at high carbon levels with sufficiently basic slag.
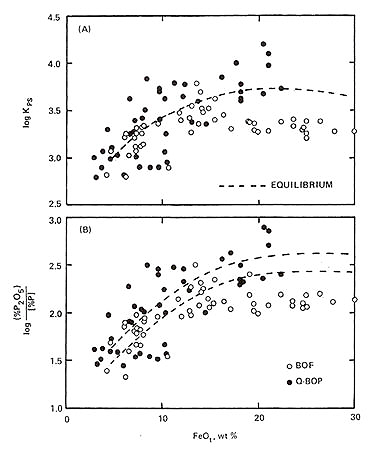
Figure 2: Effect of FeO content of slag on phosphorus distribution and log kps value
Figure 2 demonstrates phosphorus distribution dependence on slag FeO content in LD and Q-BOP processes. The parameter kps is defined as:
kps = (%P2O5)/[%P]•(1 + (%SiO2)) = φ ((%FeO),B) .....(13)
where B represents basicity; for B>2.5, kps becomes independent of basicity.
Phosphorus distribution also correlates with steel carbon content at tapping. Due to lower carbon levels achieved in bottom-blown processes, phosphorus distribution typically exceeds LD process performance. Generally, high basicity and low slag temperature, regardless of FeO content, favor dephosphorization.
Sulfur Removal Mechanisms and Optimization
Primary Sulfur Transfer Reactions
Sulfur transfer occurs through multiple mechanisms:
[S] + (O2)g = (SO2)g .....(14)
Approximately 15-25% of dissolved sulfur undergoes direct oxidation into the gaseous phase due to turbulent, oxidizing conditions in the jet impact zone.
Desulfurization Process Dynamics
In Basic Oxygen Furnaces, metal desulfurization proceeds slowly as a diffusion-controlled process. Process acceleration occurs through improved bath mixing, increased temperature, enhanced slag fluidity and basicity, and elevated sulfur activity. During initial heat stages, when metal contains high carbon and silicon levels, sulfur activity reaches maximum values. Additionally, partial sulfur removal occurs during early process stages at relatively low melt temperatures through manganese reaction:
[Mn] + [S] = (MnS) .....(15)
Increased iron oxide concentrations in slag promote lime dissolution, favoring desulfurization. However, secondary and most intensive desulfurization occurs during heat completion when lime dissolves at maximum rate and slag basicity reaches B=2.8 or higher. Total metal desulfurization depends primarily on homogeneous final slag basicity formed during the final minutes of metal blowing.
Industrial Sulfur Control Strategies
Increased slag basicity reduces residual sulfur concentration in metal bath, raising the sulfur distribution coefficient between slag and metal up to 10. Greater slag volume facilitates increased sulfur transfer at identical distribution coefficients. However, excessive slag formation proves detrimental, increasing iron losses through burning, causing splashing, and accelerating lining wear.
Steel plants employ regression equations based on operational data for end-point sulfur prediction within acceptable limits. One example equation is:
(%S)/[S] = 1.42B – 0.13(%FeO) + 0.89 .....(16)
Equation 16 demonstrates slag basicity benefits and FeO's retarding influence on sulfur distribution. Numerous similar correlations exist in literature, though they remain suitable only for specific local conditions.
Conclusion
The oxygen steelmaking process represents the cornerstone of modern steel production, utilizing controlled oxidation reactions to transform pig iron into high-quality steel. Success depends on understanding and optimizing the complex interactions between carbon removal, silicon oxidation, manganese control, phosphorus partitioning, and sulfur removal. Effective process control requires careful management of oxygen flow rates, lance positioning, slag composition, and temperature profiles throughout the blowing cycle.
Access Precise Properties of Structural Steels Now!
Total Materia Horizon contains property information for 150,000+ structural steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.