Strengthening Mechanisms of Metals: Part Two
Abstract
This article examines four critical strengthening mechanisms in metals: precipitation hardening, fiber strengthening, point defect strengthening, and martensite strengthening. Precipitation hardening involves solution treatment and controlled aging to form fine precipitates that impede dislocation movement. The process is demonstrated through heat-treatable aluminum alloys (2024, 6061, and 7075), which achieve optimal strength through carefully controlled heat treatments. Fiber strengthening incorporates high-strength fibers into ductile matrices to create materials with exceptional strength-to-weight ratios. Point defect strengthening utilizes vacancies and interstitials to pin dislocations, while martensite strengthening employs diffusionless phase transformations. Understanding these mechanisms enables engineers to select appropriate strengthening strategies for specific applications, balancing strength improvements with other critical properties such as ductility, weldability, and corrosion resistance.
Understanding Precipitation Hardening in Metal Alloys
Precipitation hardening, also known as age hardening, represents one of the most effective methods for strengthening metal alloys. This process involves solution treating and quenching an alloy where a second phase exists in solid solution at elevated temperatures but precipitates during quenching and subsequent aging at lower temperatures. The fundamental requirement for successful precipitation hardening is that the second phase must demonstrate high solubility at elevated temperatures while exhibiting decreasing solubility as temperature decreases.
The distinction between precipitation hardening and dispersion hardening lies in their structural characteristics. In precipitation hardening systems, atomic matching or coherency typically exists between the precipitate and matrix lattices. Conversely, dispersion-hardened systems generally lack coherency between second-phase particles and the matrix, with the second phase showing minimal solubility even at elevated temperatures.
Heat-Treatable Aluminum Alloys and Their Applications
Pure aluminum's inherent softness limits its structural applications, necessitating alloying with various elements to enhance corrosion resistance, inhibit grain growth, and increase strength. Optimal aluminum strengthening occurs through alloying and heat treatments that promote small, hard precipitate formation, which effectively interferes with dislocation movement.
Heat-treatable aluminum alloys achieve strengthening through precipitate formation, while many such alloys sacrifice weldability because welding destroys the carefully developed microstructure. The precipitation hardening process begins by heating the alloy into the single-phase region, dissolving all precipitates. Rapid quenching creates a supersaturated solid solution while trapping excess vacancies and dislocation loops that serve as nucleation sites for subsequent precipitation.
Precipitates can form through natural aging at room temperature or artificial aging at elevated temperatures, typically between 100°C and 200°C. The hardening degree depends on precipitate size, number, and relative strength, factors determined by alloy composition, tempering temperature, and tempering time.
Commercial Aluminum Alloy Systems
1. 2024-T351 (Al-4.5Cu-1.5Mg-0.6Mn) represents one of the most widely used high-strength aluminum alloys. While offering excellent formability in the annealed condition, it exhibits poor weldability common to many heat-treatable alloys. Natural aging produces GP zones consisting of copper and magnesium atoms on specific crystallographic planes, while artificial aging creates finely dispersed Al₂CuMg precipitates. Over-aging results in incoherent precipitates, and long-term dimensional stability suffers due to continued aging.
2. 6061-T651 (Al-1Mg-0.6Si-0.25Cu-0.2Cr) stands as the most popular 6000 series alloy, offering moderate strength with excellent weldability compared to other heat-treatable alloys. It demonstrates superior corrosion resistance and high plane strain fracture toughness. This alloy naturally ages to an essentially stable T4 condition, with softer conditions preservable through refrigeration. Formability in the annealed condition can be maintained for two hours at room temperature, two days at 0°C, and seven or more days at -7°C.
3. 7075-T651 (Al-5.6Zn-2.5Mg-1.6Cu-0.3Cr) exhibits very high strength and hardness with general corrosion resistance similar to 2024. However, it loses its strength advantage over 2024 at elevated temperatures while maintaining excellent plane stress and plane strain fracture toughness. Despite high static strength, fatigue resistance remains limited due to progressive breakdown of hardening particles on slip planes, which become progressively smaller until they redissolve into the matrix.
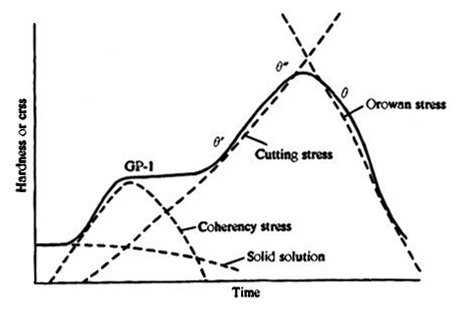
Figure 1: Interplay of various precipitation hardening mechanisms to successive stages in the hardness-time curve
Fiber Strengthening for High-Performance Applications
Fiber strengthening produces materials with exceptional strength and strength-to-weight ratios by incorporating fine fibers into ductile matrices. Success requires fibers with high strength and elastic modulus combined with ductile, non-reactive matrices. While whiskers of materials such as Al₂O₃ have shown good results due to their high strength, most fiber-strengthened materials utilize boron, graphite fibers, or metal wires such as tungsten. These fibers may be continuous or discontinuous, depending on application requirements.
Both metals and polymers serve as matrix materials, with glass-fiber-reinforced polymers representing the most common fiber-strengthened materials. A crucial distinction between fiber-strengthened and dispersion-strengthened metals is that high-modulus fibers essentially carry the entire load in fiber strengthening systems. The matrix functions to transmit loads to fibers, protect fibers from surface damage, and separate individual fibers while blunting cracks arising from fiber breakage.
Unidirectional fiber arrays create highly anisotropic materials that display shear coupling, meaning axial stress produces shear strains while shear stress generates axial strains. Unlike isotropic materials where uniaxial loads produce only axial and transverse normal strains, fiber-reinforced materials generate additional shear strains. Cross-ply laminates with different fiber orientations in each layer compensate for these effects, though they complicate composite material design.
Point Defect Strengthening Mechanisms
Point defect strengthening utilizes vacancies and interstitials to enhance material strength. Fundamental experiments involving aluminum single crystal quenching from near melting point demonstrated critical resolved shear stress increases from 5 to 50 MPa due to quenched-in vacancies. Quench-hardened crystals exhibited coarse slip bands compared to soft, slow-cooled crystals, explained by excess vacancy migration to dislocations, pinning them similarly to solute atoms.
High-energy radiation creates interstitials and vacancies through fast-moving atomic particle collisions with solid metals. Neutron irradiation significantly affects mechanical properties, increasing yield stress by factors of 2 to 4 in annealed metals. Face-centered cubic metals like aluminum and copper develop sharp yield points after irradiation, while body-centered cubic metals such as steel and molybdenum frequently lose their yield points.
Martensite Strengthening in Steel Systems
Martensite strengthening through austenite-to-martensite transformation via diffusionless shear-type transformation during steel quenching represents one of the most common strengthening processes in engineering materials. While martensitic transformations occur in various metallurgical systems, only iron-carbon-based alloys demonstrate such pronounced strengthening effects.
This strengthening mechanism also characterizes Al-Cu alloys, commercially known as aluminum bronze, extending the application of martensitic strengthening beyond traditional steel systems.
Optimizing Slip Characteristics for Enhanced Properties
Both solid solution and particle strengthening significantly influence alloy deformation characteristics. Slip process character importantly affects tensile ductility, strain-hardening rate, fatigue crack initiation, and fatigue crack growth rate. Strength increases not accompanied by improvements in these structure-sensitive properties may prove inadequate for engineering applications.
Slip character classification includes planar or wavy slip and coarse or fine slip categories. Fine wavy slip produces the most homogeneous deformation, generally yielding optimal ductility at given strength levels. Solid solution additions typically promote planar slip by reducing stacking-fault energy, making cross-slip difficult. Solutes promoting short-range order cause coarse planar slip.
Fine particle strengthening can produce planar-to-wavy or wavy-to-planar slip mode transitions depending on dislocation-particle interaction nature. Particles sheared by dislocations tend to produce coarse, planar slip, while particles bypassed by dislocations lead to fine wavy slip, optimizing the balance between strength and ductility in engineering applications.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.