Soldering of Copper and Copper Alloys: Part One
Abstract
This comprehensive article explores soldering techniques for copper and copper alloys, distinguishing between soft soldering (below 350°C) and hard soldering (higher melting points). It details various solder types including tin-lead, tin-antimony-lead, tin-zinc, lead-silver, tin-antimony, and tin-silver alloys, discussing their composition, properties, applications, and limitations. The article emphasizes the importance of proper solder selection based on mechanical requirements, temperature resistance, and environmental considerations, highlighting the growing trend toward lead-free alternatives for health and safety compliance.
Introduction to Soldering Techniques
Soldering is a versatile joining method that uses a filler metal (solder) to connect two metals without heating them to their melting points. This technique is invaluable for Steelworkers as it provides a simple and efficient way to join sheet metal, make electrical connections, and seal seams against leakage. Soldering can be effectively used with numerous metals including iron, nickel, lead, tin, copper, zinc, aluminum, and many alloys.
Soldering applications fall into two main classifications based on the melting temperature of the solder used:
- Soft soldering – uses alloys melting below 350°C
- Hard soldering – employs stronger alloys with higher melting points
For copper and copper alloys specifically, hard solders are often referred to as silver solders. Soft soldering with tin-based solders is widely used for joining copper and brass in applications where mechanical strength is not critical. In electrical applications, tin-lead solders remain the most common choice, though lead-free alternatives are increasingly specified for plumbing applications involving potable water due to health concerns. Indeed, concerns about lead poisoning are now extending beyond plumbing to other applications.
Soldering Equipment and Preparation
A soldering copper (commonly called a soldering iron) consists of a forged copper head attached to an iron rod with a handle. The handle, typically made of wood or fiber, is either forced or screwed onto the rod.
Soldering heads are available in various shapes to accommodate different applications:
- Pointed copper heads are designed for general soldering work
- Stub copper heads are used for flat seams requiring significant heat
- Bottom copper heads are ideal for reaching difficult seams in containers such as pails, pans, and trays
Proper maintenance of soldering equipment is essential. Coppers must be filed and retinned after overheating or whenever they lose their solder coating. The procedure for filing and tinning a copper includes:
- Heating the copper to a cherry red color
- Clamping the copper in a vice
- Filing the copper with a single-cut bastard file
Types of Soft Solders and Their Applications
Industry uses many different types of solder, available in various forms including bars, wires, ingots, and powders. Wire solders can be found with or without a flux core. This section covers the most commonly used solders in steelworking applications.
Tin-Lead Solder
Tin-lead alloys constitute the largest group of solders in use today. These alloys offer good corrosion resistance and can join most metals. They demonstrate excellent compatibility with soldering processes, cleaning methods, and most types of flux. When describing these solders, industry convention states the tin content first—for example, a 40/60 solder contains 40% tin and 60% lead.
The melting characteristics of tin-lead alloys depend on the ratio of tin to lead. Higher tin content results in lower melting temperatures, improved wetting ability, and reduced cracking potential.
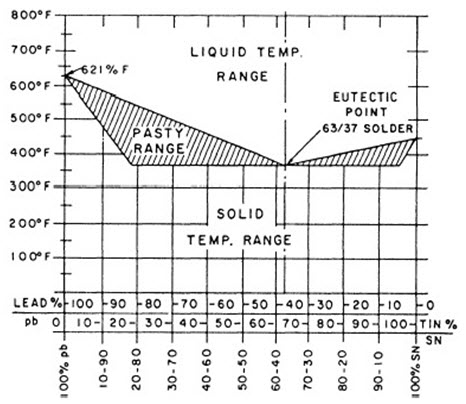
Figure 1: Tin-lead alloy constitutional diagram – this diagram shows the melting behavior of tin-lead solders, illustrating that 100% lead melts at 621°F while 100% tin melts at 450°F
As shown in Figure 1, solders containing 19.5% to 97.5% tin remain solid until they exceed 360°F. The eutectic composition for tin-lead solder is approximately 63% tin and 37% lead, which becomes completely liquid at 361°F.
Solders with lower tin content are more economical and primarily used for sheet metal products and other high-volume applications. High tin solders find extensive use in electrical work. Solders containing 60% tin or more, known as fine solders, are used in precision instrument soldering where temperature control is critical.
Tin-Antimony-Lead Solder
Antimony can be added to tin-lead solder as a partial substitute for tin. Adding antimony (up to 6%) increases the strength and mechanical properties of the solder. However, caution must be exercised when using solders with high antimony content—they should not be applied to aluminum, zinc, or zinc-coated materials. This is because they form an intermetallic compound of zinc and antimony that causes the solder to become extremely brittle.
Tin-Zinc Solder
Several tin-zinc solder formulations have been developed specifically for joining aluminum alloys. The 91/9 and 60/40 tin-zinc solders are suitable for higher temperature ranges (above 300°F), while the 80/20 and 70/30 tin-zinc alloys are typically used as precoating solders.
Lead-Silver Solder
Lead-silver solders are valuable in applications requiring strength at moderately high temperatures. Pure lead cannot be used alone because it does not normally wet steel, cast iron, copper, or copper alloys. The addition of silver to lead creates alloys that more readily wet steel and copper surfaces.
Standard lead-silver solders exhibit rather poor flow characteristics and are susceptible to humidity damage and corrosion during storage. These limitations can be addressed by introducing a small tin content (approximately 1%), which enhances wetting and flow characteristics while increasing resistance to corrosion.
It's important to note that lead-silver solders require higher soldering temperatures and special fluxing techniques. The use of a zinc-chloride base flux or uncoated metals is recommended, as rosin fluxes decompose rapidly at high temperatures.
Tin-Antimony Solder
Tin-antimony solders are specifically formulated for refrigeration work or for creating joints between copper and cast iron. The most common formulation is the 95/5 solder (95% tin, 5% antimony).
Tin-Silver Solder
Tin-silver solder (96/4) is the preferred choice for food or beverage containers because it contains no cadmium or lead, making it safe for food contact. It can also serve as an effective replacement for tin-antimony solder (95/5) in refrigeration applications.
Find Instantly Thousands of Welding Materials!
Total Materia Horizon contains thousands of materials suitable for welding and electrodes, with their properties in bulk and as welded conditions.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.