Production of Stainless Steels: Part Three
Abstract
Vacuum oxygen decarburization (VOD) represents a revolutionary time and cost-saving method for producing high-quality stainless, corrosion-resistant, and heat-resistant steels that are challenging to manufacture using conventional techniques. This advanced secondary metallurgy process enables the economical production of high-chromium steels with exceptionally low carbon, nitrogen, and hydrogen levels. The VOD process, developed in Germany between 1962-1967, accounts for approximately 98% of vacuum steel production globally. By combining vacuum conditions with oxygen refining, manufacturers achieve superior steel cleanliness, precise compositional control, and minimal chromium oxidation losses while producing extra-low carbon nitrogen (ELCN) grades with C+N content below 150 ppm.
Secondary Metallurgy in Modern Stainless Steel Production
The evolution of secondary metallurgy has transformed stainless steel manufacturing, particularly through vacuum oxygen decarburization technology. This innovative approach combines the benefits of vacuum conditions with oxygen refining to overcome traditional limitations in stainless steel production. The process addresses the fundamental challenge of decarburizing chromium-rich steels without significant chromium losses through oxidation.
Stainless steel production requires careful management of chromium, a strong oxide-forming element that complicates conventional refining processes. Traditional methods struggle to achieve sufficiently low carbon levels while preventing chromium oxidation to the slag phase. Modern secondary refining equipment, primarily AOD furnaces and VOD furnaces, solves this challenge by manipulating the refining atmosphere to ensure preferential decarburization in chromium's presence.
The key principle involves decreasing the partial pressure of carbon monoxide in the refining atmosphere. AOD furnaces achieve this through argon dilution, while VOD furnaces utilize pressure reduction techniques. This approach enables the production of specialty grades that would be impossible or economically unfeasible using conventional methods.
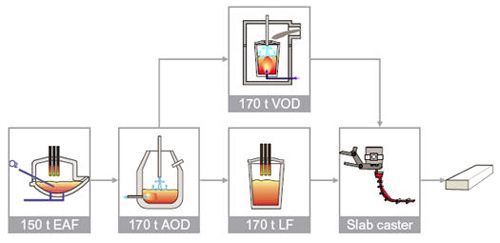
Figure 1: A typical stainless steel production facility
The VOD Process: Revolutionary Vacuum Decarburization Technology
The Vacuum Oxygen Decarburization (VOD) process emerged as the dominant vacuum production method, accounting for approximately 98% of all vacuum steel manufacturing. Developed at Witten (Thyssen) in Germany between 1962 and 1967, this groundbreaking technology revolutionized the production of high-quality specialty steels.
The VOD process offers several distinctive advantages that set it apart from conventional converter processes. Minimal argon consumption represents a significant economic benefit, requiring only about 1 cubic meter per ton of steel produced. The elimination of nitrogen pickup during tapping, which commonly occurs in converter processes, results from using the VOD ladle as the casting ladle. Additionally, silicon consumption in VOD operations remains remarkably low at just 3 kg/ton.
However, the VOD charge requirements necessitate specific compositional parameters: maximum 0.3% carbon content and less than 0.1% silicon. To minimize chromium losses in the arc furnace, an additional 3 kg/ton of silicon is typically required, bringing total silicon consumption to approximately 6 kg/ton.
Comparing VOD Process Advantages and Limitations
While the VOD decarburization process offers numerous benefits, understanding its limitations provides a complete operational perspective. The process demonstrates higher refractory consumption compared to modern converters, along with lower productivity rates in both electric furnaces and VOD units. Electric furnace processing time reduces by 25% versus the 50% reduction achievable with converter processes, while VOD charge-to-tap time extends to 50-70 minutes compared to 40-60 minutes in AOD operations.
Operational flexibility regarding charge mix compositions is somewhat limited. The VOD process requires electric furnace carbon content around 0.3% versus 1.8% for conventional methods, silicon content below 0.1% versus 0.3%, and demonstrates reduced desulfurization capability and lower scrap melting capacity. Maintenance and operating costs associated with steam production also present economic considerations.
Despite these limitations, the VOD process excels in specific applications where its advantages significantly outweigh drawbacks. The economic production of extra-low carbon nitrogen (ELCN) grades with C+N content below 150 ppm represents a primary strength. The process accommodates varying initial carbon contents, minimizes chromium oxidation losses through low CO partial pressure, and achieves high chromium recovery rates through optimized slag metallurgy.
Advanced VOD Refining Treatment Procedures
The VOD refining treatment follows a carefully orchestrated sequence that maximizes process efficiency and product quality. Initial preparation involves adjusting the required argon purging rate before closing the tank and reducing pressure to approximately 100 mbar. The oxygen lance moves into working position, directing the oxygen blow onto a slag-free spot on the steel surface.
The relationship between initial carbon content and bath level determines the optimal blowing rate, which typically varies between 0.25 and 0.7 Nm³/t per minute. Process monitoring relies on tank pressure changes and exhaust gas analyzer data, with operators controlling the reaction by adjusting lance distance, oxygen blow rate, and argon purging parameters.
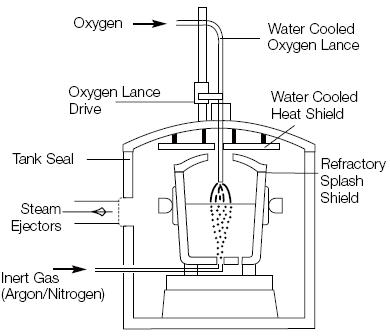
Figure 2: Vacuum Oxygen Decarburization (VOD) unit
Carbon monoxide reaction monitoring utilizes waste gas analysis, specifically measuring the CO/CO₂ ratio to track process progression. The refining treatment progresses through three distinct stages, each characterized by specific chemical reactions and gas composition changes.
Three-Stage VOD Process Optimization
The VOD process stages represent carefully controlled phases that optimize decarburization while minimizing chromium losses. During the first stage, only silicon undergoes oxidation, establishing baseline conditions for subsequent reactions. The second stage demonstrates a marked increase in the CO/CO₂ ratio, indicating strong carbon monoxide reaction activity and active decarburization.
The third stage signals process completion through changing curves that indicate increased CO₂ in waste gas, representing the end of primary refining reactions. At this point, carbon content typically reaches approximately 0.08% while chromium oxidation remains limited to 0.7-1.0%.
To prevent over-refining and excessive chromium oxidation, operators shut off oxygen supply at the optimal moment. Additional vacuum pump stages engage to create the low vacuum conditions necessary for continued decarburization using oxygen dissolved in the bath/slag system for an additional 5-30 minutes.
Process duration depends on initial carbon content and selected oxygen blow rate, while the second decarburization phase timing considers the desired final nitrogen content. Temperature rise during the exothermic refining phase can be calculated accurately using start temperature, initial carbon and silicon contents, and oxygen blow endpoint data. Normal temperature increases range between 80°C and 150°C.
Post-Refining Treatment and Quality Control
Following the second decarburization phase, the tank undergoes flooding procedures before analysis and temperature sampling. Predetermined reducing and alloying materials are added along with lime and fluorspar, with lime quantities calculated to ensure slag basicity of at least 2.5. Silicon additions include amounts required for deoxidation and reduction purposes, calculated to meet upper specification limits while ensuring complete deoxidation and superior steel cleanliness.
The melt receives additional treatment under reduced pressure after reducing agent addition. Reduction reactions proceed rapidly as lime dissolves quickly under vacuum conditions, simultaneously avoiding hydrogen pickup from reducing agents. Sulfur content reduction below 0.005%S occurs without difficulty, producing "white" slag free from metallic oxides.
Actual chromium oxidation percentages remain lower than theoretical calculations due to reversion reactions occurring during vacuum refining of iron and manganese in the slag. Further analysis correction may be necessary after the reduction phase, with teeming temperature adjusted through same-grade cooling scrap addition.
Specialized Applications: Super Ferritic Grades and SS-VOD
Titanium-stabilized grades require specific handling procedures, including melt deslagging prior to titanium addition. Homogenization follows titanium addition through brief argon stirring periods.
The production of super ferritic grades containing 29% chromium with C+N summation below 150 ppm requires specialized treatment procedures. Since nitrogen solubility decreases with increasing carbon content, initial carbon for the refining phase increases to approximately 2% carbon. Treatment ladles equipped with three inert gas purging plugs intensify bath agitation.
Oxygen blowing during vacuum refining continues until 0.02% carbon content is achieved, reducing the initial nitrogen range of 0.02-0.04% to 0.001-0.004%. During the second decarburizing phase under further reduced vacuum, carbon content of the over-refined melt decreases below 0.005%.
These ultra-low carbon melts demonstrate high susceptibility to carbon and nitrogen pickup, making alloying material selection critical. Teeming must occur under inert gas shrouding to maintain product integrity. This intensive argon purging process is known as Strong-Stirring VOD (SS-VOD), representing the pinnacle of vacuum decarburization technology.
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.