Production of Stainless Steel: Part One
Abstract
In the history of research and development of stainless steels, most important has been the decarburization of high chromium molten steel. Efficient decarburization and the prevention of chromium oxidation losses have been central to the development of steelmaking technology for stainless steels.
Because the strong affinity of chromium for carbon, all pneumatic processes for refining stainless steel attempt to achieve selective oxidation of carbon in the presence of chromium by raising the reaction temperature and/or reducing the partial pressure of CO by vacuum or mixed gas (argon-oxygen) refining.
In the history of research and development of stainless steels, most important has been the decarburization of high chromium molten steel. Efficient decarburization and the prevention of chromium oxidation losses have been central to the development of steelmaking technology for stainless steels.
Process Development
Prior to the late 1940s, stainless steel was made in the electric arc furnace by melting carbon steel scrap, iron ore and burnt lime; slagging off; adding burnt lime, ferrosilicon and fluorspar; increasing the temperature; and adding low carbon ferrochrome to attain the specified chromium. In this process (known as the "Rustless Process"), most stainless grades were made to an aim carbon specification of 0.08 percent. During this period, the only practical and economic way to use stainless scrap was to remelt it in induction furnaces. With the introduction of tonnage oxygen in the steel industry in the late 1940s, a new arc furnace practice evolved in which stainless scrap, high carbon ferrochrome, nickel, and lime were melted, the bath blown with oxygen to temperatures between 1850-1950°C, reduced with silicon and aluminum, and trimmed with low carbon ferrochrome. Relative to the “Rustless Process,” this practice resulted in significant decreases in power consumption, process time, and the use of expensive low carbon ferrochrome as well as improved quality (decreased hydrogen contents) and increased chromium recoveries.
In 1954, W. Krivsky of Union Carbide was studying carbon-chrome-temperature relationships in ferrochromium melting in the laboratory. The experiments involved blowing oxygen onto the surface of molten chromium alloy baths under isothermal conditions. In order to control temperature, he added argon and found that he could decarburize the melt to low carbon levels without excessive chromium oxidation. Krivsky's observation led to plant-scale experiments injecting argon/oxygen mixtures by lance into the arc furnace between 1958 and 1962. Eventually, it was concluded that a separate refining vessel (duplex process) was necessary to develop a commercial process, and the first successful AOD (argon oxygen decarburization) heat was made in October 1967 in a modified 15-ton ladle. These trials resulted in the first commercial AOD installation at Joslyn (now Slater Steel) in July l968.
During the late 1950s, vacuum degassing processes (DH, RH, ASEA-SKF, Finkl VAD, etc.) were developed for carbon steel production, and led to the development of vacuum decarburization processes for stainless steels in the mid-1960s. The VOD (vacuum oxygen decarburization) process was developed by Witten (now Thyssen) in Germany between 1962 and 1967. Between 1959 and 1962, Witten had produced stainless steels in their LD converter (decarburization of pig iron, addition of low carbon ferrochrome, and reduction with silicon and aluminum).
With the installation of their ladle vacuum degassing unit in 1962, they began trials duplexing premelt from the LD to the vacuum degasser. Initially, iron ore was used for decarburization. Later, premelting took place in the arc furnace, and oxygen was top blown onto the bath surface in the degassing ladle.
The AVR process, in which oxygen was injected below the surface by top lance in the degassing ladle, was developed by Allegheny Ludlum in the mid-1960s. In recent years, Allegheny has used their TMBI (top mixed bottom inert) process metal supplied from their induction furnaces when stainless demand exceeded AOD capacity. The development of the AOD and VOD processes between 1954 and 1968 revolutionized stainless steelmaking and was the primary impetus for the dramatic growth in production between 1970 and the present. The use of these or similar processes resulted in significant decreases in raw material costs, increases in productivity, and improved quality.
The Figure 1 shows some of steelmaking processes for production of stainless steels.
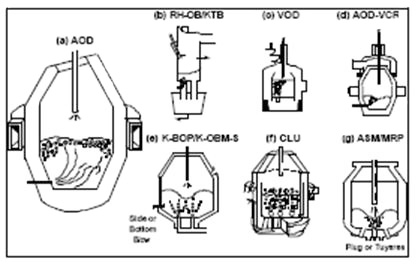
Figure 1: The Steelmaking Processes for Stainless Production (a) AOD (b) RH-OB/KTB (c) VOD (d) AOD-VCR (e) K-BOP/K-OBM-S (f) CLU (g) ASM/MRP Side or Bottom Blow Plug or Tuyeres
Theory of Iron-Chromium-Carbon Equilibria
Because the strong affinity of chromium for carbon, all pneumatic processes for refining stainless steel attempt to achieve selective oxidation of carbon in the presence of chromium by raising the reaction temperature and/or reducing the partial pressure of CO by vacuum or mixed gas (argon-oxygen) refining.
The competing reactions,
[C] + [O] = CO (g) (1)
[Cr] + [O] = Cr oxide (2)
can be treated by rigorous thermodynamic calculations only when simplifying assumptions are made in regard to the chromium oxide activity of the slag.
There has been much controversy in the literature concerning the nature of these pure oxide phases in equilibrium with iron-chromium alloys. When the metal contains less than 5% chromium, there is little doubt that the phase is chromite, FeCr2O4, and that at higher chromium levels Cr3O4 separates first. This is shown in Figure 2.
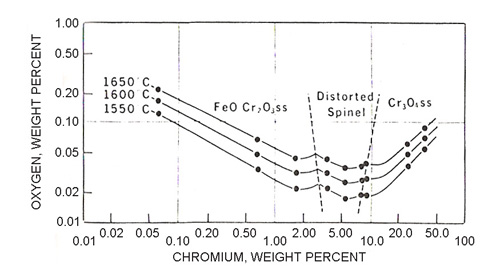
Figure 2: Oxygen solubility curves for iron-chromium alloys
The first detailed study of iron-chromium-carbon equilibria was conducted by Richardson and Dennis, who investigated the equilibrium between CO and CO2 mixtures in the presence of carbon dissolved in pure molten iron and in liquid iron-chromium alloys. Based on their data, Chipman calculated the interaction parameter eCC i.e., the effect of increased concentration of carbon on its activity coefficient, to be eCC= 358/T, where T is the absolute temperature in Kelvin. The value of eCC has more recently been calculated by Sigworth and Elliott to be eCC = 158/T + 0.0581. Similarly, eCrC, the effect of chromium on carbon activity coefficient, was found to vary between 0.020 and 0.024, depending on temperature.
The equilibrium between chromium and carbon can be described by combining two reactions of (1) and (2). For all stainless grades where Cr3O4 is the equilibrium phase according to Hilty et. al., this becomes
¼ Cr3O4 + [C] = ¾ [Cr] + CO (g) (3)
At equilibrium,
K = {PCO [aCr]¾} / {[aC] [aCr3O4]¼} (4)
where ai refers to activity of i component and K the equilibrium constant given as
log K = - 11520 /T + 7.64 (5)
Thermodynamic activity, ai, deviates from the Henry’s law can be expressed in terms of concentration as
ai = fi · [% i] (6)
For a multi-component system where the effects of each of the other components are significant, the activity coefficient, fi can be expressed as
log fi = Σ eji [% i] (7)
where the interaction parameter, eji, represents the effect of the alloying element, j, on the activity coefficient of i component. The values of the interaction coefficient of the most common alloying elements as compiled by Elliot are listed in Table 1.
Table 1: The values of the interaction coefficient of the most common alloying elements
| Dissolved elements (i) | Alloyed Elements (j) | |||||||||||
| % | Al | C | Cr | Co | Cu | Mn | Mo | Nb | Ni | O | Si | Ti |
| C | 0.043 | 0.14 | -0.024 | 0.0076 | 0.016 | -0.012 | 0.0083 | 0.06 | 0.012 | 0.34 | 0.08 | |
| Cr | 0.12 | 0.0003 | -0.019 | 0.016 | 0.0018 | 0.0002 | 0.14 | 0.0043 | 0.059 | |||
| O | 3.9 | 0.45 | -0.04 | 0.008 | -0.013 | -0.021 | 0.0035 | 0.14 | 0.006 | 0.02 | -0.131 | -0.6 |
The chromium-carbon-temperature relationship for a metal bath in equilibria with a chromium saturated slag can be derived by combining equations (4) and (7) with aCr3O4 = 1.0 as follows:
K = {PCO [aCr]¾} / {[aC] [aCr3O4]¼}= - 11520/T+7.64+(358/T+0.075)· [%C] – 0.02 [%Cr] (8)
If alloy steel grades with less than 5% Cr are to be produced, FeCr2O4 becomes the primary oxide phase. Hence, the following equilibrium equation can be derived similarly:
K = {PCO [aCr]¾} / {[aC] [aCr3O4]¼}= - 10830/T+7.06+(358/T+0.05)· [%C] – 0.02 [%Cr] (9)
The effect of additional alloying elements such as nickel and manganese can readily be accounted for by the use of the interaction coefficients listed in Table 1.
For practical application, Hylty, Rassbach and Crafts simplified the thermodynamic expression for use in the concentration range of 3 to 30% chromium:
log[%Cr]/[%C] = -13800/T + 8.76 (10)
This equation, which has been widely adopted in evolution of electric furnace practices, is very good approximation of thermodynamic equilibrium in the temperature range of 1700–1815°C (3100–3300°F) and for concentrations of from 10% to 17% chromium typical of electric furnace practice. However, over wider temperature and composition ranges encountered in the steelmaking techniques, the deviations of this equation become increasingly important. Hilty et al. also pointed out that manganese could be considered approximately similar in its effect to chromium.
Simkovich and McCoy modified equation (10) to apply to austenitic stainless steel:
log[%Cr]/[%C] = -{13800/(T + 4.21 [%Ni])} + 8.76 (11)
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.