High-Temperature Corrosion: Part Two
Abstract
Vapor species that form in any given high-temperature corrosion situation often have a strong influence on the rate of attack, the rate generally being accelerated when volatile corrosion products form.
Although most metals and alloys form a layer of oxide in contact with air, the differences in oxide growth rate can be significant.
Vapor species that form in any given high-temperature corrosion situation often have a strong influence on the rate of attack, the rate generally being accelerated when volatile corrosion products form. Gulbransen and Jansson have shown that metal and volatile oxide species are important in the kinetics of high-temperature oxidation of carbon, silicon, molybdenum, and chromium.
Six types of oxidation phenomena were identified:
1. At low temperature, diffusion of oxygen and metal species through a compact oxide film
2. At moderate and high temperatures, a combination of oxide film formation and oxide volatility
3. At moderate and high temperatures, the formation of volatile metal and oxide species at the metal-oxide interface and transport through the oxide lattice and mechanically formed cracks in the oxide layer
4. At moderate and high temperatures, the direct formation of volatile oxide gases
5. At high temperature, the gaseous diffusion of oxygen through a barrier layer of volatilized oxides
6. At high temperature, spalling of metal and oxide particles.
Corrosion kinetics
Although most metals and alloys form a layer of oxide in contact with air, the differences in oxide growth rate can be significant. For engineering purposes the differences are vital since they determine the component life-time. A linear growth rate implies that the rate of oxidation remains constant during exposure. Linear oxidation rates can be observed during the initial exposure, before a continuous oxide film has formed. A similar behaviour is also seen when a bare metal surface is revealed due to severe oxide spallation.
According to the linear rate equation described in Equation 6, the exposure time does not affect the oxidation rate and it is reactions at the surface or phase boundary that limit the oxidation rate.
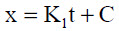 ... (6)
... (6)
Where x represents the oxide thickness, K1 the linear rate constant, t denotes time and C is a constant.
During exposure of metals and alloys suitable for use at high temperatures, the transport of reactants across the oxide scale is usually the rate-determining step and the oxidation rate decreases as the oxide thickens. In a model developed by Wagner the diffusion of ions and electrons are limiting the oxidation rate, leading to a parabolic growth rate as shown in Equation 7.
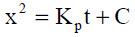 ... (7)
... (7)
Where x represents the oxide thickness, KP the parabolic rate constant, t denotes time and C is a constant.
Plots showing oxide growth rates are commonly used when assessing alloy performance during exposure in high temperature atmospheres. With a double logarithmic plot the constants of the rate equation can be easily determined. In reality this often results in a growth rate that deviates from the existing models. The obvious reason is that these are idealized models that cannot perfectly describe oxide growth on engineering alloys when several factors are simultaneously influencing the oxidation process.
The presence of fast diffusion paths at localized positions (grain boundaries, lattice defects etc.) both within the oxide and in the metal virtually means that the scale growth rate varies over the surface. Localized spallation may also occur which governs a rapid oxide growth at the position where the oxide film has ‘spalled off’. Despite discrepancies from the described growth models, a diffusion controlled oxidation is desired and commonly observed for engineering alloys. Severe cracking or spallation of the oxide film is also an important feature which results in a rapid increase in corrosion rate.
Read more
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.