Hafnium alloys: Part One
Abstract
Pure hafnium is a lustrous, silvery metal that is not so ductile nor so easily worked as zirconium The electric conductivity is about 6% that of copper. It has excellent resistance to a wide range of corrosive environments.
Hafnium is a heavy, steel-gray metal in the reactive metals group that is very closely related to zirconium, and forms a continuous solid-solution at all concentrations of zirconium and hafnium. Hafnium occurs naturally with zirconium at a ratio of approximately 1:50, and is produced exclusively as a co-product of nuclear-grade zirconium. Hafnium has an extremely high affinity for oxygen, nitrogen, and carbon, and is one of the most effective solid-solution strengtheners via dispersion strengthening. Like the other reactive metals, hafnium is HCP (Hexagonal Close-packed) at room temperature and anisotropic.

Pure hafnium is a lustrous, silvery metal that is not so ductile nor so easily worked as zirconium; nevertheless, hafnium can be hot- and cold-rolled on the same equipment and with similar techniques as those used for zirconium. All zirconium chemicals and alloys may contain some hafnium, and hafnium metal usually contains about 2% zirconium. The melting point, 2222°C, is higher than that of zirconium, and heat-resistant parts for special purposes have been made by compacting hafnium powder to a density of 98%. The metal has a close-packed hexagonal structure. The electric conductivity is about 6% that of copper and has excellent resistance to a wide range of corrosive environments.
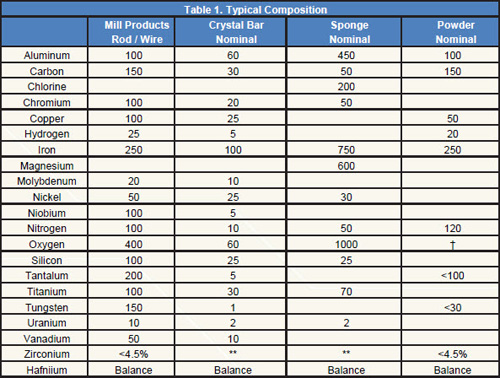
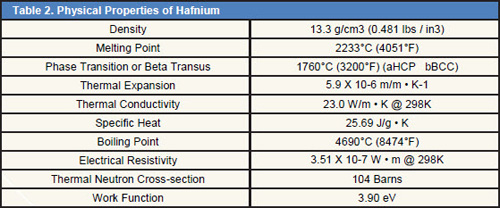
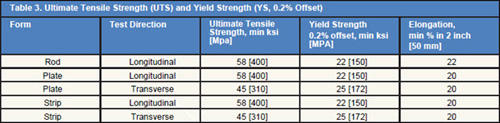
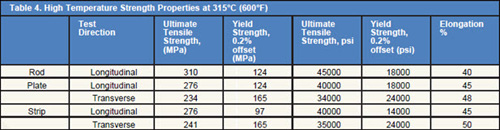
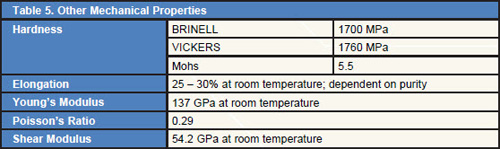
Tables 1÷5 shows the common properties of Hafnium.
Read more
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.