Corrosion Resistance of Ferritic Stainless Steels
Abstract
Ferritic stainless steels offer exceptional corrosion properties, including resistance to chloride stress-corrosion cracking, oxidizing aqueous media corrosion, high-temperature oxidation, and pitting corrosion in chloride environments. These steels contain approximately 13% or more chromium and precipitate a prime phase within the 350°C to 540°C temperature range, with maximum effects occurring at 470°C. Advanced manufacturing techniques such as vacuum and argon-oxygen decarburization have enabled the development of high-chromium ferritic steels like ASTM types 409 and 439. These materials demonstrate superior atmospheric corrosion resistance and stress-corrosion cracking resistance compared to austenitic steels, making them suitable for automotive, industrial, and marine applications where conventional stainless steels prove inadequate or uneconomical.
Understanding Ferritic Stainless Steel Composition and Properties
Ferritic stainless steels demonstrate remarkable corrosion resistance characteristics that make them valuable in numerous industrial applications. These specialized alloys contain above approximately 13% chromium and precipitate a prime phase within the 350°C to 540°C temperature range, with the maximum effect occurring at approximately 470°C. The precipitation hardening process significantly lowers temperature ductility, which must be carefully considered during both processing and usage of ferritic stainless steels, particularly those with higher chromium content.
The microstructures of these steels remain completely ferritic at both room and elevated temperatures through strategic alloying additions. Manufacturers achieve this stability by adding titanium or columbium, melting to very low levels of carbon and nitrogen, or employing both approaches. These carefully controlled microstructures provide enhanced ductility and superior corrosion resistance in weldments. Molybdenum additions significantly improve pitting corrosion resistance, while silicon and aluminum enhance resistance to high-temperature oxidation.
Advanced Manufacturing Techniques for High-Chromium Ferritic Steels
The development of newer ferritic stainless steels with high chromium content has become possible through advanced manufacturing techniques including vacuum and argon-oxygen decarburization, electron-beam melting, and large-volume vacuum induction melting. These technological advances have enabled the production of superior alloys such as ASTM designations 409 and 439, which offer enhanced performance characteristics.
Type 409 ferritic stainless steel contains 12% chromium and provides relatively low cost combined with excellent formability and weldability. However, the recommended thickness is limited to approximately 3.8 mm maximum when a ductile-to-brittle transition temperature (DBTT) at room temperature or lower is required. The atmospheric corrosion resistance of Type 409 proves adequate for functional applications, making it suitable for automobile exhaust equipment, radiator tanks, catalytic reactors, containerization, and dry fertilizer transport systems.
Type 439 ferritic stainless steel contains 18-20% chromium and demonstrates excellent resistance to chloride stress-corrosion cracking. Its resistance to general and pitting corrosion approximates that of austenitic types 304 and 316. This grade performs exceptionally well in equipment exposed to aqueous chloride environments, heat transfer applications, condenser tubing for fresh water power plants, food-handling applications, and water tubing for domestic and industrial buildings. Sheet thickness limitations require staying below approximately 3.2 mm when DBTT at room temperature or lower is necessary.
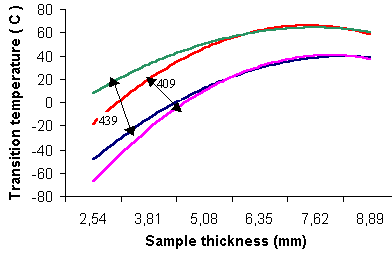
Figure 1: Ductile-to-brittle transition temperatures (DBTT) for ferritic stainless steel rising with section thickness; data scatter bands are displayed for types 409 and 439.
Superior Stress-Corrosion Cracking Resistance
The most significant advantage of ferritic stainless steels lies in their exceptional resistance to stress-corrosion cracking. These alloys demonstrate outstanding resistance to both chloride and caustic stress-corrosion cracking, significantly outperforming many alternative materials. However, nickel and copper residuals can reduce the stress-corrosion resistance of these steels, requiring careful control of these elements during production.
The susceptibility of ferritic stainless steels to intergranular corrosion results from chromium depletion caused by precipitation of chromium carbides and nitrides at grain boundaries. Due to lower solubility for carbon and nitrogen and higher diffusion rates in ferrite, the heat-affected zones of welds in ferritic stainless steels are particularly vulnerable both in the weld itself and in areas adjacent to the weld. Eliminating intergranular corrosion requires either reducing carbon to very low levels or adding titanium and columbium to bind the carbon and nitrogen.
Pitting Corrosion Resistance and General Corrosion Performance
Pitting corrosion represents an insidious localized form of corrosion occurring in halide media that can render complete installations inoperative within relatively short timeframes. Resistance to pitting corrosion depends on several critical factors including chloride concentration, exposure time, temperature, and oxygen content. Generally, pitting corrosion resistance increases proportionally with chromium content. Molybdenum additions play an equally important role, with molybdenum being equivalent to several percentage points of chromium in terms of corrosion resistance enhancement.
The atmospheric corrosion resistance of ferritic stainless steels proves excellent across diverse environmental conditions. These materials demonstrate superior corrosion resistance in strongly oxidizing environments, such as nitric acid solutions. In organic acid environments, all ferritic stainless steels perform better than austenitic alternatives. However, in reducing media, the general corrosion resistance of ferritic stainless steels typically falls short of austenitic performance.
High-Chromium Ferritic Stainless Steel Applications
High-chromium ferritic stainless steels, including types 442 and 446, exhibit exceptional resistance to both corrosion and oxidation in numerous industrial environments. These advanced alloys are included in multiple ASTM specifications including A176-74 for chromium stainless flat products, A511 for seamless stainless steel mechanical tubing, and A268-74 for ferritic stainless steel tubing for general service applications. They also meet ASME code requirements and AISI and SAE specifications.
High-chromium ferritic steels contain 18-30% chromium with carefully controlled low levels of carbon and nitrogen. Titanium additions in these alloys prevent intergranular chromium-carbide and nitride precipitation during welding or processing operations. The ferritic structure combined with controlled composition enables these alloys to exhibit superior resistance to general, intergranular, and pitting corrosion, as well as stress-corrosion cracking.
Similar to other high-chromium stainless steels, types 442 and 446 demonstrate excellent oxidation resistance at elevated temperatures. These materials also feature high thermal conductivity, higher yield strength compared to austenitic stainless steels, and lower tensile ductility characteristics.
The exceptional resistance to chlorides, organic acids, and chloride stress-corrosion cracking indicates that these high-chromium ferritic alloys are suitable for extensive applications where conventional stainless steels or alternative materials prove either inadequate or uneconomical. High-chromium ferritic stainless steels find particular utility in heat exchanger tubing, feed-water tubing, and equipment operating with chloride-bearing or brackish cooling waters.
Available in sheet, strip, tubing, and welding wire forms, these advanced alloys are finding substantial application in replacing brass and cupronickel, corrosion-resistant high-nickel alloys, and other materials throughout the food processing, power generation, chemical, petrochemical, marine, and pulp and paper industries. Their combination of superior corrosion resistance, cost-effectiveness, and mechanical properties makes them increasingly attractive for demanding industrial applications.
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.