Corrosion and Corrosion Properties of Stainless Steels: Part One
Abstract
Stainless steels owe their corrosion resistance to a spontaneous, thin oxide film that forms on their surfaces, significantly reducing corrosion rates. Chromium is the key alloying element responsible for this passive film, typically requiring about 17% chromium for effective protection. Other elements like molybdenum, nickel, and nitrogen enhance corrosion resistance in specific environments. Despite their robustness, stainless steels can suffer from localized corrosion such as pitting, especially in chloride-rich environments. This article explores the mechanisms behind stainless steel passivity, the effects of alloy composition on corrosion resistance, and common corrosion types affecting stainless steels, with emphasis on uniform and pitting corrosion. Applications and environmental considerations are also discussed to highlight material selection criteria.
Introduction: The Nature of Stainless Steel Corrosion Resistance
Stainless steels represent the most diverse and complex family of steels, prized primarily for their corrosion resistance. Their "stainless" nature arises from a protective surface layer that forms spontaneously and dramatically slows corrosion. Under typical conditions, this passive layer self-heals rapidly after damage, enabling stainless steels to potentially survive for millions of years if only uniform corrosion occurred.
Passivity and the Role of Chromium
The corrosion resistance of stainless steels depends on the formation of a very thin, invisible oxide film called the passive film. This film forms in oxidizing environments and protects the steel from aggressive chemical attack. Chromium is the most critical alloying element in stainless steels, as adding chromium causes a rapid reduction in corrosion rate—often down to about 10% of that of plain carbon steel—due to this passive film formation.
A chromium content of approximately 17% is generally necessary to develop a compact and continuous passive film, which explains why many stainless steels contain 17-18% chromium.
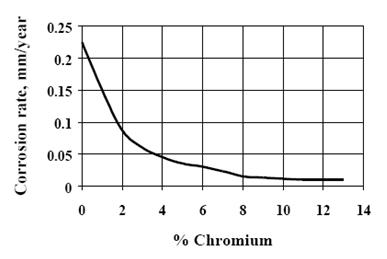
Figure 1: The effect of chromium content on passivity illustrates the significant drop in corrosion rate as chromium increases
Other alloying elements like molybdenum, nickel, and nitrogen further enhance corrosion resistance in specific environments. For example, copper improves resistance in sulfuric acid, while silicon, cerium, and aluminum contribute to high-temperature corrosion resistance in some gases.
Conditions for Maintaining Passivity
For a stainless steel to maintain its passive film, it must be oxidized. More aggressive environments require a higher supply of oxidizing agents to sustain passivity. Fortunately, stainless steels have such a strong tendency to passivate that only small amounts of oxidizing species—such as those present in air and water—are sufficient.
Unlike paint or coatings, the passive film is self-healing; if chemically or mechanically damaged, it can repair itself in oxidizing conditions. Stainless steels perform best in oxidizing, neutral, or weakly reducing environments and are less suitable for strongly reducing environments like hydrochloric acid.
Types of Corrosion in Stainless Steels
Although stainless steels resist corrosion well, failure of the passive film can occur either locally or uniformly through various mechanisms.
Uniform corrosion can happen in acidic or hot alkaline solutions, causing predictable, even material loss that can be accounted for in design.
Increasing chromium content improves general corrosion resistance, but some elements can hinder passivation. For instance, sulfur in solid solution impedes passive film formation and is generally undesirable for corrosion resistance, although it improves weldability and machinability. Welding sulfur-containing steels modifies weld pool surface tension and geometry, with grades like 316L specifying sulfur limits to balance corrosion and fabrication needs.
Nickel notably enhances corrosion resistance by promoting passivation. Consequently, austenitic stainless steels, which contain nickel, generally exhibit superior corrosion resistance compared to martensitic or ferritic grades that lack nickel, especially against mineral acids.
Pitting Corrosion: Mechanism and Influencing Factors
Pitting corrosion arises from localized breakdown of the passive film, often in chloride, halide, or bromide environments. A defect or flaw in the passive layer exposes the underlying steel to dissolution, leading to a build-up of positively charged metal ions (M⁺) and attracting negatively charged chloride ions (Cl⁻) near the site.
This localized chemistry lowers the pH dramatically (to around 2 or 3), preventing repassivation of the film and accelerating pit growth. Current densities inside pits can reach above 1 A/cm² compared to nanoampere/cm² in passive areas. Chloride ion concentration inside pits can be thousands of times higher than in the surrounding solution.
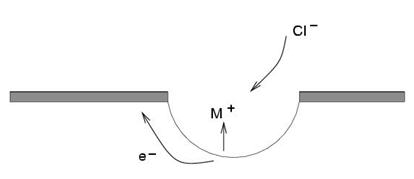
Figure 2: Schematic illustration of pitting corrosion
The key chemical reactions involved in pitting corrosion are:
Anodic dissolution:
M → M+ + e−
Metal chloride formation and hydrolysis:
M+ + Cl−+ H2O → MOH + H+ + Cl−
(This reaction causes the pH to drop locally.)
Cathodic reaction near the pit surface:
O2 + 2H2O + 4e− → 4OH−
The initiation mechanism of pitting remains debated but is often linked to manganese sulfide (MnS) inclusions surrounded by chromium-depleted zones, which may trigger pit formation.
Alloy Composition and Pitting Resistance
Pitting resistance improves with increased chromium content and the addition of molybdenum and nitrogen, though their effects vary in potency. To quantify this, a pitting resistance index is used:
Pitting index = Cr + 3.3 × Mo + 16 × N
where Cr, Mo, and N are weight percentages.
Marine environments are a common concern for pitting corrosion. Austenitic stainless steel AISI 316, with about 18% Cr, 12% Ni, and 2-3% Mo, is widely used due to good pitting resistance. More severe environments, such as offshore platforms, require highly alloyed steels with up to 6% molybdenum.
Street furniture in colder climates exposed to de-icing salts also demands materials with strong pitting resistance.
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.