Corrosion Behavior of Aluminum in Chloride Mediums
Abstract
This study investigates the corrosion resistance of aluminum alloys (AlMn and sandwich material AlSi/AlMn/AlSi) in chloride-containing environments using electrochemical techniques. The research examines how varying chloride concentrations (100, 300, and 500 ppm) affect the polarization resistance of these materials over time. Results demonstrate that both alloys exhibit good corrosion resistance in Glaceol RX D cooling liquid, even with chloride additions. The sandwich material AlSi/AlMn/AlSi consistently outperformed the uncovered AlMn alloy across all test conditions. These findings highlight the importance of using demineralized water when diluting antifreeze liquids to maintain optimal corrosion protection for aluminum components in automotive cooling systems.
Introduction to Aluminum Corrosion Resistance
Aluminum is among the most widely used corrosion-resistant metals in industrial applications. Its excellent resistance stems from the hard, tough oxide film naturally formed on its surface. However, if this protective oxide layer becomes compromised through scratching or amalgamation, aluminum becomes vulnerable to attack by hot alkali hydroxides, halogens, various non-metals, and even water under ordinary conditions.
The corrosion behavior of aluminum has been extensively studied due to its significance in contemporary industrial applications. Research has established that there exists a potential region where corrosion rates remain minimal, even in aggressive media such as chloride solutions. The relatively complex corrosion mechanism of aluminum has been examined by numerous researchers, who have determined that corrosion occurs only when the metal's protective oxide layer is damaged and when the natural repair mechanism is prevented by chemical dissolution.
Electrochemical Techniques for Corrosion Analysis
Polarization methods have been widely employed to investigate the mechanisms of localized corrosion and the processes leading to it. Using potentiostatic techniques, researchers can vary the potential to study different aspects of corrosion behavior. Both potentiostatic and potentiodynamic techniques have been applied by several researchers to study aluminum corrosion in various environments.
The exceptional corrosion resistance of aluminum in many environments can be attributed to its protective oxide film, which remains relatively inert chemically and provides the passive behavior characteristic of aluminum.
Research Focus and Methodology
Constantin F. and colleagues studied two materials: an AlMn alloy and a sandwich material AlSi/AlMn/AlSi. Their primary objective was to determine how chloride concentration influences the corrosion resistance of these materials.
These aluminum alloys are commonly employed in automotive cooling systems, particularly for radiators, where they are exposed to cooling liquids often containing various salts, including ammonium or potassium chloride. The chloride anion is frequently responsible for the breakdown of the passive layer that forms spontaneously on aluminum surfaces, which otherwise efficiently protects the material.
For this study, three chloride concentrations were selected: 100, 300, and 500 ppm. The research was conducted at room temperature using various electrochemical techniques.
Table 1. Nominal chemical composition (wt.%) of investigated materials
| Designation | Si | Fe | Cu | Mn | Mg | Other | Al | ||
| AlMn | 0.2 | 0.4 | 0.15 | 1.05 | 0.003 | 0.4 | Remaining | ||
| Sandwich material | |||||||||
| AlMn | 0.24 | 0.55 | 0.066 | 1.15 | 0.03 | 0.4 | Remaining | ||
| AlSi | 9.25 | 0.37 | 0.002 | 0.003 | 0.001 | 0.4 | Remaining | ||
Results of Polarization Resistance Testing
Figure 1a shows the evolution of polarization resistance versus time for the AlMn alloy in Glaceol RX D with different additions of Cl-ions (100, 300, and 500 ppm NaCl). The corrosion experiments were conducted after stabilization of the free corrosion potential. Polarization resistance measurements were taken after immersion in the electrolyte solution at 1h, 3h, 9h, and 19h, with resistance values calculated using QuickCalc software M352, considering only the linear zone of the curve.
For solutions containing 100 or 300 ppm NaCl, the polarization resistance increased rapidly during the first half of the immersion period, reaching 130-165 kOhms.cm². However, after 9 hours of immersion, a significant decrease of approximately four orders of magnitude was observed, indicating an increase in corrosion rate. In the 500 ppm NaCl medium, polarization resistance remained constant throughout the experiment, with values slightly lower than those measured for 300 ppm NaCl at the end of the test (19h).
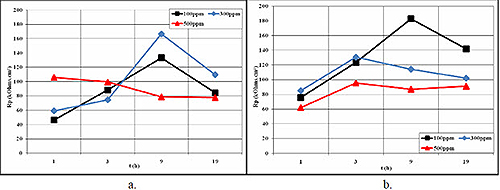
Figure 1a & 1b: Evolution of polarization resistance versus time in Glaceol RX D with different additions of Cl- ions: a) AlMn and b) AlSi/AlMn/AlSi
Figure 1b illustrates the evolution of polarization resistance versus time for the AlSi/AlMn/AlSi alloy in Glaceol RX D with the same chloride ion concentrations. The curve for the medium containing 100 ppm NaCl shows similar behavior to that recorded for AlMn under the same conditions. For the other two NaCl concentrations (300 and 500 ppm), the curves remain almost constant over time, with polarization resistance values of approximately 100 kOhms.cm² at the end of the test period.
Figure 2 compares the polarization resistance evolution versus immersion time for both materials in Glaceol RX D (with 100 and 500 ppm NaCl). The protective effect of the AlSi layer can be clearly observed when compared to uncovered AlMn. In both solutions, the corrosion rate is lower for the aluminum sandwich material, with polarization resistance values of approximately 200 kOhms.cm² for the solution containing 100 ppm NaCl, and 140 kOhms.cm² for the solution containing 500 ppm NaCl.
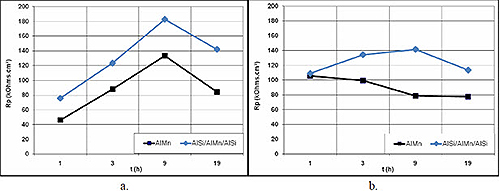
Figure 2a & 2b: Evolution of polarization resistance versus time in Glaceol RX D - comparison of the two aluminum alloys: a) with 100 ppm NaCl and b) with 500 ppm NaCl
Practical Implications for Automotive Cooling Systems
The findings from this research have direct implications for maintaining automotive cooling systems. The addition of chloride ions to the cooling liquid simulates the potential contamination that might occur when antifreeze is diluted with non-demineralized water. The results clearly indicate that such contamination can accelerate corrosion rates in aluminum components.
Conclusions
Both investigated materials, AlMn and AlSi/AlMn/AlSi alloys, demonstrated sufficiently good corrosion resistance in the Glaceol RX D cooling liquid (30 vol.%) recommended by Dacia, even with the addition of Cl- ions (100 to 500 ppm NaCl). These additions represent the possibility of Glaceol dilution in natural water supplies, which creates a more corrosive environment.
This work highlights the importance of the chloride ion concentration and, consequently, the importance of using demineralized water when diluting antifreeze liquids. Additionally, the AlSi/AlMn/AlSi sandwich material consistently exhibited better corrosion resistance than AlMn across all tested media.
Access Precise Properties of Aluminum Alloys Now!
Total Materia Horizon contains property information for 30,000+ alumiums: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.