Copper Matrix Hardening by Alumina Particles
Abstract
This article presents synthesis of the nanocomposite Cu-Al2O3 powder by thermochemical method and sintering with a comparative analysis of the mechanical and electrical properties of obtained solid samples. Nanocrystaline Cu-Al2O3 powders were produced by thermochemical method through following stages: spray-drying, oxidation of precursor powder, reduction by hydrogen and homogenization. The obtained nanocomposite, which structure is with certain changes preserved in final structure, has provided sintered material with homogenous distribution of dispersoide in copper matrix, with exceptional effects of reinforcing and excellent combination of mechanical and electrical properties.
This article presents synthesis of the nanocomposite Cu-Al2O3 powder by thermochemical method and sintering with a comparative analysis of the mechanical and electrical properties of obtained solid samples. Nanocrystaline Cu-Al2O3 powders were produced by thermochemical method through following stages: spray-drying, oxidation of precursor powder, reduction by hydrogen and homogenization. Size of produced powders was 20-50nm with noticeable presence of agglomerates. Composite powders are characterized with Al2O3 homogenous distribution in copper matrix. Powders were cold pressed with pressure of 500 MPa and sintered. Sintering of the obtained samples was performed in the hydrogen atmosphere in isothermal conditions at temperature range from 800 to 900°C and time up to 120 minutes. The obtained nanocomposite, which structure is with certain changes preserved in final structure, has provided sintered material with homogenous distribution of dispersoide in copper matrix, with exceptional effects of reinforcing and excellent combination of mechanical and electrical properties.
Oxide dispersion hardened copper processed by mechanical alloying is characterized by very fine grains (with sizes less than 100 nm in diameter) and a presence of extremely small oxide dispersions (approximately 30-40 nm in diameter). Depending on the size and interparticle distance, dispersoids drag effectively recrystalization and boundary migration at both room and elevated temperatures. It was assumed that large alumina particles could be broken down under the extreme conditions of hydrostatic compression that exist when powder particles are trapped between colliding grinding balls during mechanical alloying.
The most intensive grain refinement occurs in the early stage of milling, i.e. up to 10h, whereas with the prolonged milling time the grain size remains practically constant reaching 30nm (Cu+3wt% Al2O3) and 40nm (Cu+4wt% Al2O3) after 20h of milling. Since the increase of grain size during hot-pressing is less than 60%, it is reasonable to expect that the compacts will be characterized by a very high grain size refinement. It was confirmed that milled mixture particles demonstrate lamellar structure, typical for mechanically alloyed powders. In this case, lamellas represent individual plastically deformed copper particles with embedded alumina particles. Such a lamellar structure is retained after compaction.
The effect of commercial alumina content on microhardness of dispersion hardened copper has been investigated. Measurements of thermal stability were carried out as a function of alumina content and high temperature heat treatment at 800°C up to 5h.
- The microhardness of compacts processed from mechanically alloyed copper increases with milling time.
- The increases in microhardness were assumed to be due to the uniformly distributed alumina particles and small grain size of the copper matrix.
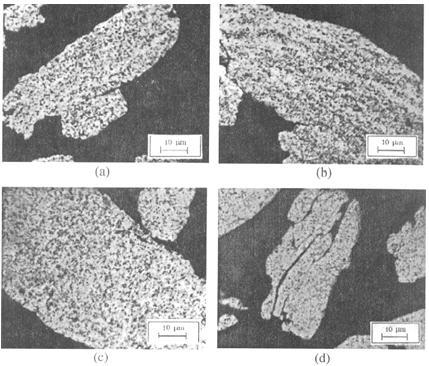
Figure 1: Distribution of alumina particles in the Cu+4mas% Al2O3 copper matrix after different milling time: a) 3h, b) 5h, c) 10h, d) 20h.
- The Cu+3wt. %Al2O3 compacts exhibit a higher increase in microhardness than Cu+4wt. % Al2O3 compacts.
- After 20h of milling the microhardness of Cu+3wt. % Al2O3 and Cu+4wt. % Al2O3 compacts was 2440 MPa and 1726 MPa, which is 3.6 and 2.6 times higher than the microhardness of as-received electrolytic copper (670 MPa) compacted under the same conditions.
- After a high temperature exposure at 800°C, the mechanically alloyed copper compacts showed a sharp decrease in microhardness becoming lower than microhradness of compacts processed from as-received electrolytic copper powder.
- The low temperature stability during high temperature exposure may be ascribed to the fact that the process of fragmentation of commercial alumina particles has not been completely achieved.
Research of nanocrystal materials in recent years has been intensified, primarily due to their attractive potential, i.e. properties which are significantly improved compared to the conventional grain materials. Nanostructure materials rank into the group of ultrafine, metastable structures which contain a high concentration of defects (point defects, dislocations) and boundaries (grain boundaries, interphase boundaries and etc). These materials are structurally different from crystals and amorphous forms, because of the fact that grain boundaries and interphases represent a specific state of the solid matter, since the atoms on the boundaries are subjected to periodical potential field of crystal from the both sides of the boundary.
Obtaining powders of metals and alloys represents a starting stage in production of sintered metal materials. For obtaining sintered products with the demanded properties, the starting material, powder of metal or alloy, has a decisive importance. Having in mind that the starting structure, although suffering certain changes in further processing, basically is preserved in the structure of the final product, a necessity of working out greater number of methods for production of powders is imposed.
Nanostructual materials can be synthesized in controlled processes by the following methods:
- condensation from the gas stage,
- by synthesis in vacuum, highly energetic reactive milling,
- by precipitation from solution (sol-gel, hydrothermal synthesis, electrochemical synthesis, reactions in aerosol-reactive spraying, sublimated drying).
Obtaining of powders by a thermochemical method, where input materials are in liquid state, is not a new procedure and recently, due to development of contemporary materials with on advanced set of properties, it came to an intensive interest in this method for production of ultra fine and nano powders. The synthesis of nanocomposite powders by a chemical method is possible in two ways. The first method comprises adding of a certain quantity of CuO into solution of aluminum nitrate. For other synthesis method aluminum nitrate and CuO are also mixed in appropriate proportion in distilled water. However, in this case, amonium hydroxide is added to form gel for hydrolysis of aluminium nitrate up to hydroxide.
In both cases, mixture was annealed at 850°C, and then reduced in hydrogen atmosphere at 975°C for 2h until the final structure was obtained. Nanopowders give better performance in sintering due to their high surface area, and therefore, can tremendously improve the sintering process. The part produced with nanopowders will have high density, hardness and fracture toughness.
Fine dispersed particles introduction into the metal matrix, have significant reinforcing effects, which can be kept at elevated temperatures. For such reinforcement, ultra fine and nano particles of oxides are suitable, which, due to their hardness, stability and insolubility in the base metal also represent obstacles to moving of dislocations at the elevated temperatures. Research of dispersion reinforced materials, points out the significance of properties of the starting powders, importance of the starting structure, respectively, which, although suffer certain changes in further processing, basically remains preserved in the structure of the final product. Also very important aspect of dispersion strengthening is introduction of as less as possible amount of dispersed particles into volume of base material.
The above characteristics significantly determine the later-stage processing and sintering properties and eventually determine the microstructure of the composite. Uniform powder shape and narrow size distribution provide reduced microstructural defects in the sintered composites by enhancing powder flow and packing efficiency during slip casting and cold isostatic pressing (CIP).
Sintering of ultradispersed powders occurs due to sliding of the particles along their borders, then to a dislocative mechanism which is responsible for creation of surplus vacancies. Concentration of surplus vacancies can reach a value which corresponds to the concentration of vacancies in the area of temperatures near to the materials melting temperature. On this basis, it could be concluded that diffusion activity during sintering of ultradispersed particles in the area of really low temperatures (0.1-0.3Tm) is conditioned by presence of unbalancing "recrystallization" vacancies. High recrystallization rate of ultradispersed particles are a consequence of the selfactivated recrystallization process.
Detailed characterization of the obtained powder which comprised DT/GA, XRD and AEM, points out possibility of synthesis of nanocomposite Cu-Al2O3 system by a thermochemical method, by deposition from aqueous solutions of metal salts, Cu(NO3)2 and Al(NO3)3. The microstructure of the obtained powder clearly indicated particles of 20-50nm size.
Such obtained nanocomposite powders, with the structure preserved in the structure of the final product, have provided production of the sintered system with exceptional effects of reinforcement and a good combination of mechanical and electrical properties. Thereby, the results of the hardness examination of the sintered samples show that the growth of the hardness value is in function of decreasing specific electric resistance, i.e. structural stabilization of the system, which was confirmed by the microstructural research. The achieved significant effects of reinforcement are consequence of relatively even distribution of Al2O3 in the copper metal matrix, already achieved in the process of powder synthesis by depositing from the liquid phase, which is also confirmed by the results of the sample surface analysis by EDS.
Access Precise Properties of Copper Alloys Now!
Total Materia Horizon contains property information for 30,000+ copper alloys: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.