Copper and Copper Alloys
Abstract
Copper and its alloys represent one of the most versatile and widely used groups of commercial metals in modern industry. These materials are prized for their exceptional electrical and thermal conductivity, superior corrosion resistance, ease of fabrication, and excellent mechanical properties including strength and fatigue resistance. This article explores the fundamental properties of copper and its various alloy families, including electrical coppers, dilute copper alloys, brasses, bronzes, copper nickels, and nickel silvers. It examines the effects of alloying elements on copper's properties, manufacturing processes like cold working, hot working, and annealing, and highlights specialized applications ranging from electrical components to corrosion-resistant systems. The article also discusses the metallurgical principles behind different strengthening mechanisms and the role of deoxidizers in copper alloy production.
Fundamental Properties and Applications of Copper
Copper and its alloys constitute one of the major groups of commercial metals. They are widely used because of their excellent electrical and thermal conductivity, outstanding resistance to corrosion, and ease of fabrication, together with good strength and fatigue resistance. They are generally nonmagnetic.
They can be readily brazed, and many coppers and copper alloys can be welded by various gas, arc and resistance methods. For decorative parts, standard alloys having specific colors are readily available. Copper alloys can be polished and buffed to almost any desired texture and luster. They can be plated, coated with organic substances or chemically colored to further extend the variety of available finishes.
Versatile Applications Across Industries
Pure copper is used extensively for cables and wires, electrical contacts, and a wide variety of other parts that are required to pass electrical current. Coppers and certain brasses, bronzes and cupronickels are used extensively for automobile radiators, heat exchangers, home heating systems, panels for absorbing solar energy and various other applications requiring rapid conduction of heat across or along a metal section. Because of their outstanding ability to resist corrosion, coppers, brasses, some bronzes, and cupronickels are used for pipes, valves and fittings in systems carrying potable water, process water or other aqueous fluids.
In all classes of copper alloys, certain alloy compositions for wrought products have counterparts among the cast alloys, which enables the designer to make an initial alloy selection before deciding on the manufacturing process.
Most wrought alloys are available in various cold worked conditions, which have room temperature strengths and fatigue resistances that depend on the amount of cold work more than on alloy content. Typical applications of cold worked conditions (cold worked tempers) include springs, fasteners, hardware, small gears, and cams. Certain types of parts - most notably plumbing fittings and valves - are produced by hot forging simply because no other fabrication process can produce the required shapes and properties as economically.
Copper alloys containing 1 to 6% Pb are free machining grades, and are used widely for machined parts especially those produced in screw machines.
Electrical and Thermal Conductivity Properties
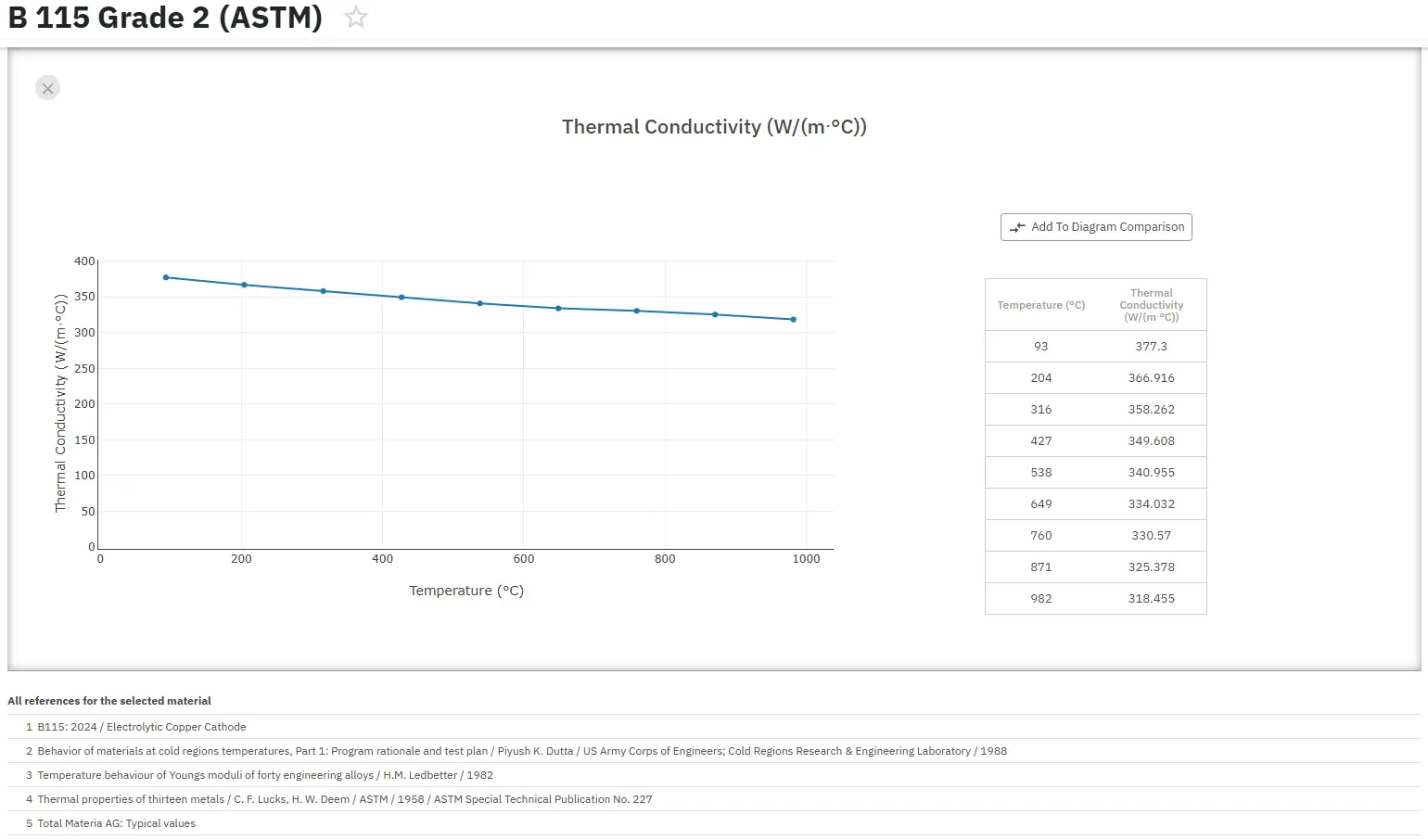
Copper and its alloys are relatively good conductors of electricity and heat. In fact, copper is used for these purposes more often than any other metal. Alloying invariably decreases electrical conductivity and, to a lesser extent, thermal conductivity. For this reason, coppers and high copper alloys are preferred over copper alloys containing more than a few percent total alloy content when high electrical or thermal conductivity is required for the application. The amount of reduction due to alloying does not depend on conductivity or any other bulk property of the alloying element, but only on the effect that the particular foreign atoms have on the copper lattice.
Electrical Coppers and Their Characteristics
Commercially pure copper is represented by UNS numbers C10100 to C13000. The various coppers within this group have different degrees of purity, and therefore different metal characteristics. Fire refined tough pitch copper C12500 is made by deoxidizing anode copper until the oxygen content has been lowered to the optimum value of 0.02 to 0.04%.
Electrolytic tough pitch copper C11000 is made from cathode copper - that is, copper that has been refined electrolytically. C11000 is the most common of all the electrical coppers. It has high electrical conductivity, in excess of 100% IACS. It has the same oxygen content as C12500, but differs in sulfur content and in over-all purity. C11000 has less than 50 ppm total metallic impurities (including sulfur).
Specialized Copper Types and Processing Methods
Oxygen-free coppers C10100 and C10200 are made by induction melting prime-quality cathode copper under nonoxidizing conditions produced by a granulated graphite bath covering and a protective reducing atmosphere that is low in hydrogen.
If resistance to softening at slightly elevated temperature is required, C11100 is often specified. This copper contains a small amount of cadmium, which raises the temperature at which recovery and recrystallization occurs.
High purity copper is a very soft metal. It is softest in its undeformed, single-crystal form, requiring a shear stress of only 3.9 MPa. Annealed tough pitch copper is almost as soft as high purity copper, but many of the copper alloys are much harder and stiffer, even in annealed tempers.
Cold working increases both tensile strength and yield strength, but the effect is more pronounced on the latter. For most coppers and copper alloys, the tensile strength of the hardest cold-worked temper is approximately twice the tensile strength of the annealed temper. For the same alloys, the yield strength of the hardest cold worked temper may be as much as five to six times that of the annealed temper.
Hot working - Not all shaping is confined to cold deformation. Hot working is commonly used for alloys that remain ductile above the recrystallization temperature. Hot working permits more extensive changes in shape than cold working, so that a single operation can replace a sequence of forming and annealing operations.
Thermal Processing and Specialty Copper Alloys
Annealing - Work-hardened metal can be returned to a soft state by heating, or annealing. During annealing, deformed and highly stressed crystals are transformed into unstressed crystals by recovery, recrystallization and grain growth. In severely deformed metal, recrystallization occurs at lower temperatures than in lightly deformed metal. Also, the grains are smaller and more uniform in size when severely deformed metal is recrystallized. Grain size can be controlled by proper selection of cold working and annealing practices.
Anneal-resistant coppers - Addition of small amounts of elements such as silver and cadmium to deoxidized copper increase resistance to softening at times and temperatures encountered in soldering operations such as those used to join components of automobile and truck radiators. The thermal and electrical conductivity of copper are relatively unaffected by small amounts of either silver or cadmium. Room temperature mechanical properties also are unchanged. C11100, C14300 and C16200 (cadmium-bearing coppers) work harden at higher rates than either C11400 or C11000.
Copper Alloy Families and Their Properties
The most common way to catalog copper and its alloys is to divide them into six families: coppers, dilute copper alloys, brasses, bronzes, copper nickels and nickel silvers. The first family, the coppers, is essentially commercially pure copper, which ordinarily is soft and ductile and contains less than about 0.7% total impurities. The dilute copper alloys contain small amounts of various alloying elements that modify one or more of the basic properties of copper.
Metallurgical Principles of Copper Alloys
Solid Solution Alloys - The most compatible alloying elements with copper are those that form solid-solution fields. These include all elements forming useful alloy families (Zn, Sn, Al, Si…). Hardening in these systems is great enough to make useful objects without encountering brittleness associated with second phases or compounds.
Cartridge brass is typical of this group, consisting of 30% Zn in copper and exhibiting no beta phase except an occasional small amount due to segregation, which normally disappears after the first anneal. Provided that there are no elements such as Fe, cold working and grain growth relationships are easily reproduced in practice.
Age-hardenable - Age hardening produces very high strengths, but is limited to those few copper alloys in which the solubility of the alloying element decreases sharply with decreasing temperature. The beryllium coppers can be considered typical of the age-hardenable copper alloys. Other age-hardenable alloys include C15000 (zirconium copper); C18200, C18400 and C18500 (chromium coppers); C19000 and C19100 (copper nickel phosphorus alloys); and C64700 (copper nickel silicon alloy).
By combining cold working with heat treatment, higher strengths can be obtained than can be achieved by either cold working or age hardening alone. Beryllium copper illustrates well the effects of heat treatment and cold working: in the soft, solution treated condition, the tensile strength is about 500 MPa, solution treated and aged, about 1000 MPa, and solution treated, cold worked and aged, about 1400 MPa.
Some age-hardening alloys have different desirable characteristics, such as high strength combined with better electrical conductivity than the beryllium coppers.
Specialized Alloying Elements and Deoxidation Processes
Insoluble Alloying Elements - Lead, tellurium and selenium are added to copper and its alloys to improve machinability. They, along with bismuth, make hot rolling and hot forming nearly impossible and severely limit the useful range of cold working.
An exception here are the high-zinc brasses, which become fully beta phase at high temperature. The beta phase can dissolve lead, thus avoiding a liquid grain-boundary phase at hot forging or extrusion temperatures. Most free-cutting brass rod is made by beta extrusion. C37700, one of the leading high-zinc brasses, is so readily hot forged that it is the standard alloy against which the forgeability of all copper alloys is judged.
Deoxidizers - Li, Na, Be, Mg, B, Al, C, Si and P can be used to deoxidize copper. Ca, Mn and Zn can sometimes be considered deoxidizers, although they normally fulfill different roles.
The first requirement of a deoxidizer is that it have an affinity for oxygen in molten copper. Probably the second most important requirement is that it be relatively inexpensive compared to copper and any other additions. Thus, although zinc normally functions as a solid-solution strengthener, it is sometimes added in small amounts to function as a deoxidizer, because it has high affinity for oxygen and is relatively low in cost. In tin bronze, phosphorus has traditionally been the deoxidizer, hence the name "phosphor bronzes" for these alloys. Silicon instead of phosphorus is the deoxidizer for chromium coppers because phosphorus severely reduces electrical conductivity. Most deoxidizers contribute to hardness and other qualities, which often makes classification as a deoxidizer indistinct.
Access Precise Properties of Copper Alloys Now!
Total Materia Horizon contains property information for 30,000+ copper alloys: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.