Influence of Alloying Elements on Steel Microstructure
Abstract
This comprehensive study examines the complex relationship between alloying elements and steel microstructure, challenging traditional assumptions about their properties. The research explores how various elements influence steel's characteristics beyond simplistic attributions, demonstrating that elements like Chromium, Nickel, and Manganese have context-dependent effects. The article details the classification of alloying elements as austenite-forming, ferrite-forming, carbide-forming, and nitride-forming, emphasizing their role in phase formation and stabilization. Through detailed analysis of multi-alloyed steels and the use of Schaeffler diagrams, the study provides insights into the practical implications for steel manufacturing and heat treatment processes, offering valuable guidance for materials engineering applications.
Understanding Alloying Elements in Steel
The traditional approach to understanding alloying elements in steel has often relied on oversimplified correlations between specific elements and their perceived effects. While it has been commonly accepted that Chromium (Cr) contributes to hardness and that Nickel (Ni) and Manganese (Mn) enhance toughness, these generalizations can be misleading. The reality is more nuanced, as demonstrated by various examples. For instance, while a 2% C, 12% Cr tool steel exhibits significant hardness upon hardening, a steel containing 0.10% C and 12% Cr shows only modest hardness after the same treatment. Similarly, Manganese's effect on toughness varies significantly based on its concentration, producing different outcomes in the 1-5% range compared to its behavior in 13% Hadfield steel.
The Role of Phase-Forming Elements in Steel Microstructure
The ability of alloying elements to promote and stabilize specific phases represents a crucial aspect of steel metallurgy. These elements can be systematically categorized based on their primary influences on microstructure:
Austenite-Forming Elements: Carbon (C), Nickel (Ni), and Manganese (Mn) stand as the principal austenite-forming elements. In sufficient quantities, Ni or Mn can maintain austenitic structure even at room temperature, as demonstrated in two significant examples:
- Hadfield steel (13% Mn, 1.2% Cr, 1% C), where both Mn and C contribute to austenite stability
- Austenitic stainless steel (18% Cr, 8% Ni)
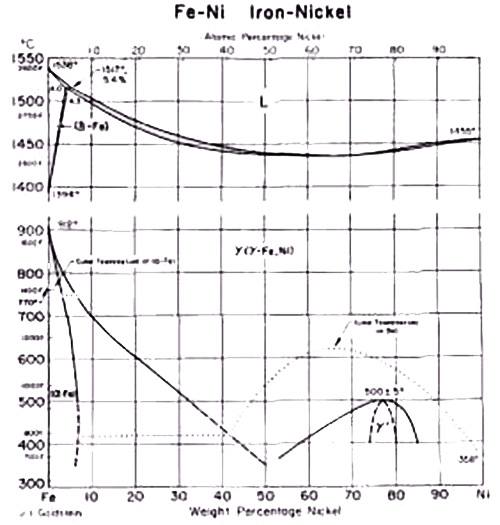
Figure 1. Fe-Ni equilibrium diagram
The Fe-Ni equilibrium diagram illustrates this relationship clearly, showing that a 10% Ni alloy achieves complete austenitic structure at 700°C. During cooling, the transformation from γ (gamma) to α (alpha) occurs between 700-300°C.
Ferrite-Forming Elements - The key ferrite-forming elements include Chromium (Cr), Silicon (Si), Molybdenum (Mo), Tungsten (W), and Aluminum (Al).
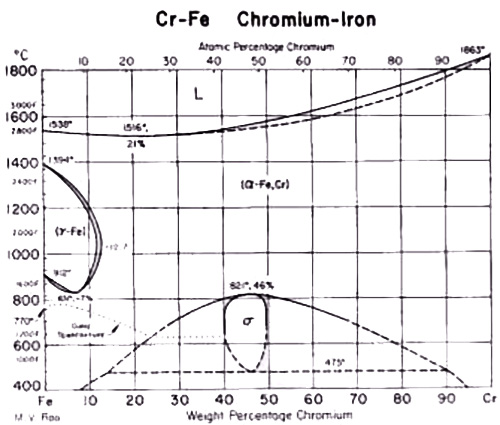
Figure 2. Cr-Fe equilibrium diagram
A particularly notable example is found in Fe-Cr alloys containing more than 13% Cr, which maintain ferritic structure at all temperatures below melting. Another practical application is seen in transformer sheet material, where a low-carbon steel with approximately 3% Si exhibits ferritic properties.
Multi-Component Steel Systems and Phase Prediction
The complexity of modern steel alloys extends beyond simple binary relationships, as most commercial steels contain three or more alloying elements. While ternary phase diagrams offer some insight into three-component systems, their practical application is limited by two key factors:
- They represent only equilibrium conditions
- Most commercial steels contain more than three components
The Schaeffler Diagram Approach: A more practical solution for understanding complex steel compositions comes through the Schaeffler diagram method.
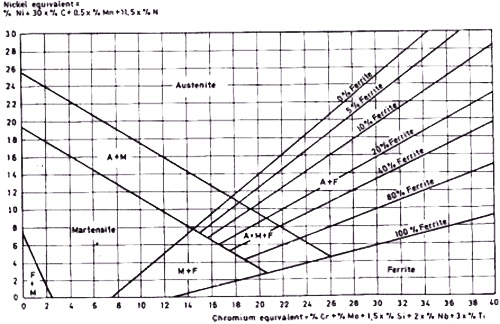
Figure 3. Modified Schaeffler diagram
This innovative approach:
- Places austenite-forming elements on the vertical axis
- Positions ferrite-forming elements on the horizontal axis
- Uses coefficient-based equivalency calculations to compare different elements
Practical Applications of the Schaeffler Method: Several industrial examples demonstrate the diagram's utility:
- Martensitic 12% Cr steel with 0.3% C (nickel equivalent of 9)
- Austenitic 18/8 steel (18% Cr, 8% Ni) with 0-0.5% C and 2% Mn
- Hadfield steel with 13% Mn, showing how carbon content affects phase stability
This section introduces more advanced concepts while maintaining clear connections to practical applications. The information has been organized to flow logically from theoretical understanding to real-world examples, with clear figure placement indicators.
Carbide Formation and Stabilization in Steel Systems
Carbide-Forming Elements and Their Characteristics: Many ferrite-forming elements also exhibit carbide-forming properties. The affinity for carbon increases progressively among these elements in the following sequence:
Cr → W → Mo → V → Ti → Nb → Ta → Zr
In steel systems, carbides manifest in two distinct categories. Special carbides, which are non-iron-containing, include compounds such as Cr7C3, W2C, VC, and Mo2C. The second category comprises double or complex carbides, which contain both iron and a carbide-forming element, with Fe4W2C serving as a notable example.
High-speed and hot-work tool steels typically exhibit three carbide types, designated as M6C, M23C6, and MC, where 'M' represents the collective metal atoms. M6C represents compounds like Fe4W2C or Fe4Mo2C, while M23C6 corresponds to Cr23C6, and MC represents carbides such as VC or V4C3.
The stability of carbides depends significantly on the presence and distribution of other elements in the steel matrix. This relationship is quantified through the partition coefficient (K), which measures the weight percentage ratio of elements between cementite and the matrix.
Table 1. Partition coefficients for elements from Al to Ta
| Al | Cu | P | Si | Co | Ni | W | Mo | Mn | Cr | Ti | Nb | Ta |
| 0 | 0 | 0 | 0 | 0,2 | 0,3 | 2 | 8 | 11,4 | 28 | Increasing | ||
In practical applications, several key observations emerge. Manganese, while inherently a weak carbide former, demonstrates remarkable effectiveness as a carbide stabilizer. Chromium maintains its position as the most commonly utilized carbide stabilizer in industrial applications. The production of malleable cast iron requires careful attention to avoid chromium content entirely. Additionally, steels containing only Silicon or Nickel show susceptibility to graphitization, a condition typically prevented through strategic chromium addition.
Nitride Formation and Surface Hardening in Steel
The relationship between carbide and nitride formation extends across alloying elements, as all carbide-forming elements also demonstrate nitride-forming capabilities. This characteristic becomes particularly significant in surface treatment processes, where nitrogen can be deliberately introduced into the steel's surface through nitriding procedures.
Surface hardness measurements of various nitrided alloy steels provide valuable insights into the behavior of different alloying elements. These elements contribute to hardness enhancement through two primary mechanisms: direct formation of hard nitrides and precipitation hardening.
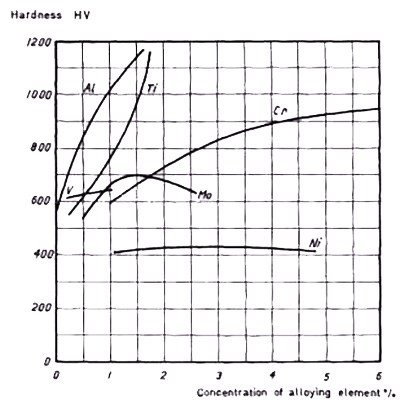
Figure 4: Graph showing the effect of alloying element additions on hardness after nitriding. Base composition: 0.25% C, 0.30% Si, 0.70% Mn
Experimental results reveal particularly interesting findings regarding hardness development. When aluminum or titanium is added to steel in concentrations of approximately 1.5%, exceptionally high surface hardness values are achieved through the nitriding process. This phenomenon demonstrates the significant impact these elements have on the nitriding response of steel.
The base material referenced in Figure 4 achieves a hardness of approximately 400 HV after nitriding. Notably, the addition of nickel to this composition shows no impact on the final hardness values, as nickel does not participate in nitride formation. This observation underscores the specificity of nitride-forming reactions and their dependence on particular alloying elements.
These findings have significant implications for industrial applications where surface hardness is crucial, such as in wear-resistant components and tooling applications. The selection of alloying elements can thus be optimized based on their nitride-forming capabilities when surface hardening treatments are planned.
Find Instantly Thousands of Metallography Diagrams!
Total Materia Horizon contains a unique collection of metallography images across a large range of metallic alloys, countries, standards and heat treatments.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.