Problems and Prospect of Al-Mg Alloys Application in Marine Constructions
Abstract
The evolution of ship performances promoted the need for the development of new Al-alloys with improved mechanical properties, corrosion resistance, weldability, etc. The non-heat-treatable Al-Mg alloys were appointed as favorable ones in respect to the costs and all the required properties for successful vessel service. The weight saving normally ranges from 50% in hulls to 62% in commercial deckhouse structures.
The evolution of ship performances promoted the need for the development of new Al-alloys with improved mechanical properties, corrosion resistance, weldability, etc. The non-heat-treatable Al-Mg alloys were appointed as favorable ones in respect to the costs and all the required properties for successful vessel service. The weight saving normally ranges from 50% in hulls (although 59% is possible in this application) to 62% in commercial deckhouse structures.
The traditional and the most often used Al-alloys in shipbuilding are 5083 type Al- Mg alloy for plates, and 6082 type Al-Mg-Si alloy for extrusions. The corrosion resistance of the 5xxx series alloys is another major factor in the selection of aluminum for marine applications.
Corrosion resistance
Carbon and low-alloy steels are not resistant to corrosion attack in aggressive environments such as seawater. They need a proper surface protection as painting or other type of coatings which should be regularly repeated. On the other hand, aluminum corrodes over 100 times slower than the carbon steels. The excellent corrosion resistance of aluminum originates from the tightly bonded oxide film formed on the surface when it is exposed to air or water. Even such a very well adhering oxide film can fail in salt water, allowing a certain degree of corrosion. The most popular aluminum alloys for use in corrosive environments are non-heat treatable 5000, and heat-treatable 6000 type alloys, because of well balanced strength parameters, weldabilty and formability. The 6000 alloys are stronger but two-three times less corrosion resistant than the 5000. The little or no need for maintenance of marine structure surfaces (less frequent painting or other coating refreshments) make an important cost saving during the service -life of any aluminum component.
High content of Mg (from 4-6 wt%), provides god strength level of standard 5083 and new developed 5383, 5383NG and 5059 Al-Mg alloys for marine application. On the other hand, benefit of increasing the strength with increasing the Mg content is followed by decreasing of corrosion resistance, due to the tendency of Mg atoms to be precipitated as highly anodic (β-phase (Mg5Al8) particles, preferably distributed along grain boundaries. In order to find out a balance between mechanical properties and corrosion resistance, an optimization of chemical composition and microstructure development during thermomechanical treatment (TMT) is necessary to be achieved.
In Al-Mg alloys with high Mg content (>3%Mg) solid solution is supersaturated with Mg solute atoms, because the Mg content is higher than 1.9%Mg, which is the equilibrium solubility of Mg in Al-matrix at room temperature. In that case, Mg solute atoms tend to precipitate out as an equilibrium β-phase (Mg5Al8) along the grain boundaries or randomly distributed in the structure. Precipitation sequences of the decomposition of supersaturated solid solution have been reported earlier as follows:
α-Al matrix → GP zones → (β'-phase → (β-phase (Mg5Al8)
This process occurs slowly even at room temperature, and could be significantly accelerated at high temperatures (>65°C). Since the corrosion potential of β-phase (-1,24V), is more negative than the potential of Al-matrix (-0.87V), dissolution of anodic (β-phase particles would occur in an appropriate solution, such as seawater. Other second phase particles, such as MnAl6, have no influence on the corrosion behavior of Al-Mg alloys, since the corrosion potential of MnAI6 particles (-0.85V) is as much as the potential of Al-matrix.
Basic forms of corrosion
The most important corrosion processes for Al-Mg alloys are:
- pitting corrosion,
- intergranular corrosion (IGC),
- stress corrosion cracking (SCC), and,
- exfoliation.
Pitting Corrosion. Pitting corrosion is very similar to crevice corrosion. Pitting of aluminum alloys occurs if the electrolyte contains a low level of chloride anions, and if the alloy is at a potential above the "pitting potential." Much research has been conducted on the pitting corrosion of aluminum. The pitting potential of unalloyed aluminum in a NaCI-H2O2 solution is approximately -0.76 versus SCE. Pitting initiates at defects on the surface of the aluminum, such as at second phase particles or on grain boundaries. The potential can be affected by having oxygen present for oxygen reduction or galvanic contact with a more noble metal, or via an applied voltage.
Intergranular Corrosion. Intergranular corrosion is a special form of corrosion characterized by the preferential attack of the grain boundaries. Intergranular (IG) corrosion is also referred to as intergranular attack (IGA). IG corrosion only occurs if the grain boundary regions are compositionally different from the bulk of the alloy. This compositional difference occurs during heat treating, aging, or welding by diffusion of atoms and precipitation of second phase particles.
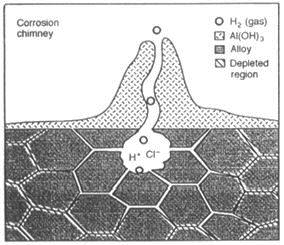
Figure 1: The pitting corrosion forms an acidic environment which leads to preferential dissolution of the grain boundary regions.
Intergranular corrosion initiates often at the sites of crevice corrosion or pitting corrosion. The acidic pit environment leads to the preferential attack of the grain boundary region, as illustrated in the schematic in Fig. 1. The two different mechanisms by which IG corrosion may occur are illustrated in Fig. 2. The first mechanism is the precipitation of a more active phase, for example Al-Mg at the grain boundaries in 5000 series alloys. On exposure to saltwater, this phase dissolves preferentially (Fig. 2(a), leaving the bulk grains separated from each other. The second mechanism of IG corrosion occurs if a more noble phase precipitates at the grain boundaries, for example CuAl2 in the 2000 or the 6000 alloys which contain copper.
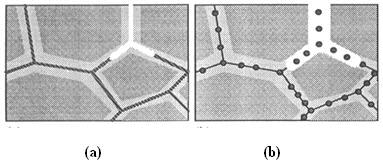
Figure 2: Intergranular corrosion can occur by either (a) the dissolution of a more active grain boundary precipitate; or (b) the dissolution of a depleted matrix around a more noble grain boundary precipitate.
Exfoliation Corrosion. Exfoliation corrosion is a special form of intergranular corrosion which occurs when the grains are flattened by heavy deformation during hot or cold rolling, and where no recrystallization has occurred. Exfoliation is characteristic for the 2000 (Al-Cu), 5000 (Al-Mg), and 7000 (Al-Zn) series alloys which have grain boundary precipitation or depleted grain boundary regions.
Stress Corrosion Cracking (SCC). Stress corrosion cracking (SCC) is the bane of aluminum alloys. Sometimes very little corrosion is evident on the outer surface, but it is possible for a critical length stress corrosion crack to be extending deep into the structure, causing a rapid and unexpected failure. In SCC, the external corrosion is minimal, but the cracks are significant. SCC requires three simultaneous conditions, first a susceptible alloy, second a humid or water environment, and third a tensile stress which will open the crack and enable crack propagation. In transgranular stress corrosion cracking (TGSCC), the cracks cut through the grains and are oblivious to the grain boundaries.
Corrosion Testing. The susceptibility to IGC of Al-Mg alloys can be determined using NAMLT test (Nitric Acid Mass Loss Test) which is described in ASTM G67 standard. It consists on the immersion of the tested specimen in concentrated HNO3 at 30°C for 24h, and measuring the mass loss. Specimens susceptible to IGC lose 25-75 mg/cm2, while the specimens resistant to IGC lose between 1-15mg/cm2.
The susceptibility to exfoliation can be determined by visual inspection using ASSET method (Method for Visual Assessment of Exfoliation Corrosion Susceptibility of AA5xxx Series Al alloys), which is described in ASTM G66 standard. It consists on the immersion of specimens in an appropriate solution at 65°C for 24h.
In order to examine SCC susceptibility, slow strain rate testing (SSRT) is recommended to be used, and it is described in ASTM G 129 standard. (Standard Practice for SSRT to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking). It consists on the slow strain rate tension test in the corrosive environment. For the assessment of SCC susceptibility, total elongations are measured, and compared with those in slow rate tension in dry air.
Corrosion prevention methods. The manufacturing process of the marine grade Al-Mg alloys needs a careful control, in order to ensure proper structure refinement, starting even in the hot rolling sequence. The basic request in manufacturing practice of Al-Mg alloys with >3%Mg for marine application, is related to the control of the precipitation of β-phase, which could be highly sensitive to the seawater attack.
Different tempers of highly alloyed Al-Mg alloys for marine application, such as H116 and H321, are developed to produce the microstructures with β-phase precipitates within the grains, in order to eliminate corrosion susceptibility. On the other hand, new Al-Mg alloys with small addition of Zn, such as 5383, 5383NG and 5059, have been designed in order to improve corrosion resistance of standard Al-Mg alloys, such as 5083 alloy.
Other methods of corrosion prevention. Aluminum anodizing is an electrochemical method of making a protective aluminum oxide film Al2O3, at the surface of Al-alloy products. Cladding is a method of coating of Al-alloys products by thin layer of pure Al or Al-alloys. If the cladding layer is anodic in comparison to the base Al alloy, the products are called alclad products. Alloy 7072 -Al-Zn is used as cladding for Al-Mg and Al-Mg-Si alloys.
The high-strength alloys are employed where welding is not required, and where their higher strengths can 'be used to advantage. Because of their lower resistance to corrosion by sea water, protective measures such as cladding, painting, or cathodic protection must be used for satisfactory life in marine service.
Access Precise Properties of Aluminum Alloys Now!
Total Materia Horizon contains property information for 30,000+ alumiums: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.