The Nitrocarburizing Process: Part One
Abstract
Nitrocarburizing, by definition, is a thermochemical treatment that is applied to a ferrous object in order to produce surface enrichment in nitrogen and carbon which in turn form a compound layer.
The composition, function and control of the furnace atmosphere are of crucial importance for the result of all hardening and thermochemical operations.
Nitrocarburizing, by definition, is a thermochemical treatment that is applied to a ferrous object in order to produce surface enrichment in nitrogen and carbon which in turn form a compound layer.
The composition, function and control of the furnace atmosphere are of crucial importance for the result of all hardening and thermochemical operations. The purpose of nitrocarburizing is to improve wear, corrosion and fatigue resistance of constructional parts. These improvements can be understood when looking at the surface microstructure and hardness after treatment, Figure 1.
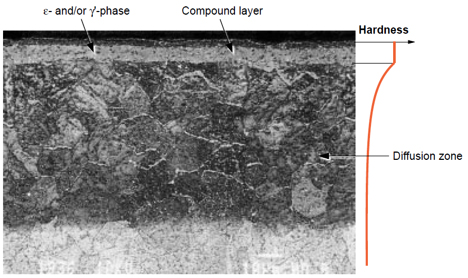
Figure 1:This micrograph shows both the compound layer and the underlying diffusion zone (darker region) in a nitrocarburized 5 % Cr tool steel (2h/580°C). The compound layer consists here of only e-phase with a thin, outer, porous zone. Scanning electron micrograph (SEM) magnification about 700 times.
The nitrocarburizing process consists of three principal steps: heating, diffusion at the nitrocarburizing temperature and cooling. Pre-heating to accelerate the nitrocarburizing process and post-oxidation to improve corrosion resistance are optional steps. Ferritic nitrocarburizing in gas is commonly carried out at temperatures ranging from 560 to 580°C.
As mentioned above, the composition of the atmosphere is a very important parameter in gaseous nitrocarburizing and needs to be closely controlled. The atmosphere consists of nitrogen (N2), ammonia (NH3), carbon dioxide (CO2) and hydrogen (H2). Ammonia is used as source of nitrogen. Carbon dioxide decomposes into carbon monoxide which together with hydrogen is needed for the transfer of carbon to the steel surface. Sometimes carbon monoxide is used directly instead of carbon dioxide. Hydrogen is not always added separately as it forms from the decomposition of ammonia. Nitrogen is used to control the percentage of the other gases and for purging the furnace before and after treatment as ammonia can form an explosive mixture with oxygen. The flow rate of different gases in each process step are plotted in Figure 2.
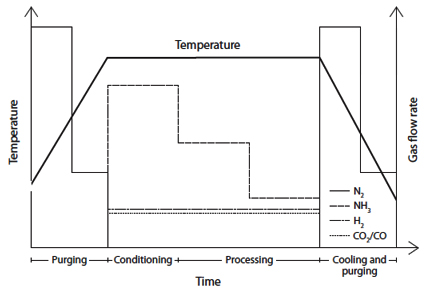
Figure 2:Gas ratio per process step.
Nitrocarburizing is widely applied in manufacturing of machine components and tools, since improved surface hardness, fatigue strength and corrosion resistance at elevated temperatures are achieved at minimal distortion. Thus, the service life of a part is significantly extended. Moreover, corrosion nonresistant steel grades gain an enhanced corrosion resistance due to the established compound zone.
Мгновенный доступ к тысячам диаграмм термической обработки!
Total Materia Horizon содержит данные по термообработке для сотен тысяч материалов: диаграммы прокаливаемости, отпуска, TTT и CCT, и многое другое.

Получите бесплатный тестовый аккаунт в Total Materia Horizon и присоединяйтесь к сообществу из более чем 500 000 пользователей из 120+ стран.