Thermodynamics of TiN Formation in Melts: Part One
Abstract
In steelmaking process, a strong nitride forming element such as titanium is often added to stabilize nitrogen and improve mechanical properties of steel through the grain refinement during hot rolling.
Recently, the formation of titanium nitride as a secondary inclusion precipitated during solidification received great interest because it is known to help the formation of equi-axed cast structure. On the other hand, titanium nitride formed in liquid steel can agglomerate and cause a nozzle clogging during continuous casting and surface quality problems of final products. In order to control the formation titanium nitride in various grades of steel, it is essential to have accurate thermodynamic data of titanium and nitrogen in steel.
Introduction
In steelmaking process, a strong nitride forming element such as titanium is often added to stabilize nitrogen and improve mechanical properties of steel through the grain refinement during hot rolling. Recently, the formation of titanium nitride as a secondary inclusion precipitated during solidification received great interest because it is known to help the formation of equi-axed cast structure. On the other hand, titanium nitride formed in liquid steel can agglomerate and cause a nozzle clogging during continuous casting and surface quality problems of final products. In order to control the formation titanium nitride in various grades of steel, it is essential to have accurate thermodynamic data of titanium and nitrogen in steels containing other alloying elements.
The reaction equilibrium for the formation of TiN in liquid iron can be written as:
TiN(S) = [Ti] + [N] .......... (1)
ΔG° = 379000 - 149 T J .......... (2)
K = hTi hN / αTiN= fTi fN[%Ti] [%N] .......... (3)
Where:
- K is the equilibrium constant for reaction 1
- hTi hN activity of titanium and nitrogen relative to 1 mass% standard state in liquid iron respectively
- fTi and fN interaction coefficient titanium and nitrogen, respectively.
During deoxidation process, the interaction parameter between titanium and silicon in liquid steels is important, because recently complex deoxidation by silicon and titanium is carried out in many grades of steels including stainless steels. The results from many investigations using similar technique show a large disagreement on the effect of silicon on the activity coefficient of titanium.
In the other hand, it is well known that the thermodynamic data of titanium in Fe-Cr melts are not consistent in the literature, and little information is available at temperatures other than 1600°C.
Because of the all mentioned above, the purpose of the investigations by J.J.Pak et. al., where to obtain reliable thermodynamic data of titanium and nitrogen/silicon in both Fe-Cr and Fe-Si melts as a function of temperature, in order to predict under what condition titanium nitride will form during steel processing. The metal-nitride-gas equilibration experiments were carried out to determine soluble titanium and nitrogen in Fe-Cr and Fe-Si melts respectively in the presence of pure solid TiN under various nitrogen pressures. The experiment was carried out in a graphite resistance furnace in the temperature range of 1570-1650°C.
Activity Coefficient of Titanium
Table 1 and 2 summarizes the experimental results of metal-nitride-gas equilibrium for Fe-Cr and Fe-Si melts at 1843-1923 K (1570-1650°C).
During the equilibration experiments, the following equilibrium can be established:
TiN(S) = [Ti] + ½N2(g) .......... (4)
ΔG° = 375 402 - 172.89 T J .......... (5)
K= hTi P½N2 / αTiN = fTi[%Ti] P½N2 .......... (6)
Where K is the equilibrium constant for reaction (1), and hTi is the activity of titanium relative to 1 mass% standard state in liquid iron, fTi is the activity coefficient of titanium and PN2 is the nitrogen partial pressure in the gas phase. Under the experimental condition, the activity of titanium nitride is unity.
Table 1: Experimental results of metal-nitride gas equilibrium in Fe-Si melts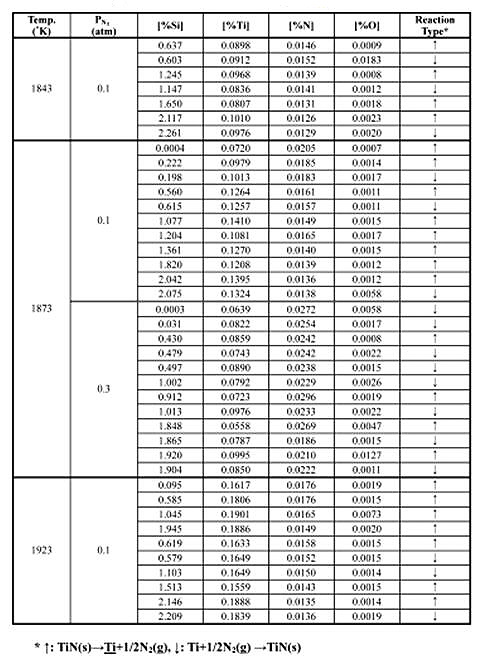
Table 2: Experimental results of metal-nitride gas equilibrium in Fe-Cr melts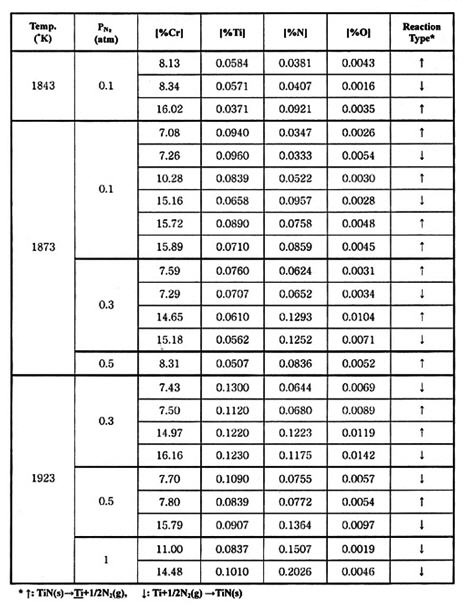
The equilibrium constant K can be rewritten as the following relation using the appropriate interaction parameters:
log KFe-Cr = log fTi + log[%Ti] + ½log PN2 =
= eCrTi[%Cr] + rCrTi[%Cr]2 + eTiTi[%Ti] + eNTi[%N] + eOTi[%O] + log[%Ti] + ½log PN2 .......... (7)
Then
log fCrTi = eCrTi[%Cr] + rCrTi[%Cr]2 =
= log K11 - eTiTi[%Ti] - eNTi[%N] - eOTi[%O] - log[%Ti] - ½log PN2 .......... (8)
Where fCrTi is the interaction coefficient of Cr on Ti in liquid iron, and eCrTi and rCrTi are the first and second order interaction parameters of Cr on Ti, respectively.
In the same way, for Fe-Si melts, the equilibrium constant K can be rewritten as the following relation using the appropriate interaction parameters:
log KFe-Si = log fTi + log[%Ti] + ½log PN2 =
= eSiTi[%Si] + rSiTi[%Si]2 + eTiTi[%Ti] + eNTi[%N] + log[%Ti] + ½log PN2 .......... (9)
Then
log fSiTi = eSiTi[%Si] + rSiTi[%Si]2 =
= log K12 - eTiTi[%Ti] - eNTi[%N] - log[%Ti] - ½log PN2 .......... (10)
Where fSiTi is the interaction coefficient of Si on Ti and is a measure of the effect of specific concentration of Si on the behavior of Ti in dilute solution in liquid iron, and eSiTi and rSiTi are the first and second order interaction parameters of Si on Ti, respectively.
The Tables 3 and 4 show the values of eCrTi; rCrTi, eSiTi; rSiTi in Eq.9 and Eq.10 given from many experimental results of equilibrium titanium and nitrogen contents as a function of both chromium content in Fe-Cr-Ti-N melts and silicon content in Fe-Si-Ti-N melts.
Table 3: Interaction parameters of Si on Ti and N
| 1873 K | Temperature Dependency |
|
| eSiTi | -0.0256 | 177.5/T-0.120 |
| 2.1 | ||
| 1.431 | ||
| 0.064 | ||
| rSiTi | 0 | |
| -0.00065 | ||
| eSiN | 0.0491 | -286.2/T + 0.202 |
| 0.047 | ||
| 0.048 | ||
| -0.038 | ||
| rSiN | 0 |
Table 4: Interaction parameters of Cr on Ti and N
| 1873 K | Temperature Dependency |
|
| eCrTi | 0.028 | 1196/T-0.61 |
| 0.055 | ||
| 0.022 | ||
| 0.016 | ||
| 0.024 | ||
| rCrTi | 0 | |
| -0.0001 | ||
| 0.0005 | ||
| -0.045 | - 850/T + 0.409 | |
| eCrN | -0.047 | |
| -0.046 | - 164/T - 0.0415 | |
| -0.045 | ||
| rCrN | 0 | |
| -0.0004 | ||
| 0.00032 | 1.67/T - 0.0006 |
Finden Sie sofort die genauen Zusammensetzungen von Materialien!
Total Materia Horizon enthält die chemischen Zusammensetzungen von Hunderttausenden von Werkstoffen und Substanzen sowie deren mechanische und physikalische Eigenschaften und vieles mehr.

Holen Sie sich ein KOSTENLOSES Testkonto bei Total Materia Horizon und schließen Sie sich einer Gemeinschaft von über 500.000 Benutzern aus mehr als 120 Ländern an.