Irradiation Effects in Nuclear Materials: Part One
Abstract
Structural materials used in Nuclear applications have a critical role to play in ensuring the safety and integrity of the construction and associated maintenance.
The main challenge is to understand the damaging effects of neutron, gamma, beta and alpha radiation and how some of these can cause significant damage to the crystalline structure of materials and then combat it with correct selection and ongoing maintenance.
Since the harnessing of the atom and the advent of nuclear power for peaceful purposes in electrical generating stations and propulsion plants, the effects of radiation on the structural materials that make up the reactor plants has played an important part in the design and construction of these nuclear reactors. It has long been known that irradiation alters the physical and mechanical materials.
However, to understand the effects of radiation, one must first be familiar with the radiations and their interaction mechanisms.
Materials in nuclear service are subjected to various types of radiation, e.g. neutron, gamma, beta and alpha. Some of these can cause significant damage to the crystalline structure of materials. Nuclear radiation focuses large amounts of energy into highly localized areas. Damage is caused by the interaction of this energy with the nuclei and/or orbiting electrons.
Gamma, beta and alpha are classed as ionizing radiation because they interact only with electrons surrounding nuclei. Ionizing radiation strips electrons from the outermost orbitals of the atoms, creating nuclei which are charged ions. This ionization disrupts the atomic bonding of materials. The stripped electrons may continue to create subsequent damage.
To explain further, it is necessary to consider the different types of atomic bonds.
Covalent bonds occur when atoms are held together by the sharing of electron pairs. Ionizing radiation, by stripping electrons from their atoms, can break up bond pairs of electrons. This causes disintegration of the original molecules and the formation of new and different ones, i.e., a chemical change. Organic compounds such as oils, plastics or rubber (natural and synthetic) contain almost exclusively covalent bonding and, therefore, suffer significant damage by ionizing radiation. Further details of this radiation damage are given later in this module.
Ionic bonds form when oppositely charged ions are held together in a crystal lattice by electrostatic attraction only; all electrons in the compound are held in their orbits around particular atoms. Ionizing radiation is not nearly so destructive to an ionic bond as to a covalent one. The electrical charge on individual ions may be altered by the stripping of electrons from an atom, but the oppositely charged pairs will still experience electrostatic attraction.
The majority of inorganic materials. other than metals, exhibit ionic bonding and are normally good insulators because all available electron sites are filled and there is very little electron movement from site to site. Since ionizing radiation causes electron migration. the resistivity of these materials is reduced during irradiation. making them more conductive.
In metallic bonding, a simple view of the bonding arrangement is most practical for considering the effects of ionizing radiation. Metals consist of positive ions held in fixed positions (a crystal lattice) surrounded by a sea of electrons. The electrons are very mobile and not attached to any particular atoms; they move from atom to atom under a variety of influences. Ionizing radiation will have only a transient effect on metals because the electrons stripped from any particular atoms are readily replaced through the free movement of other electrons.
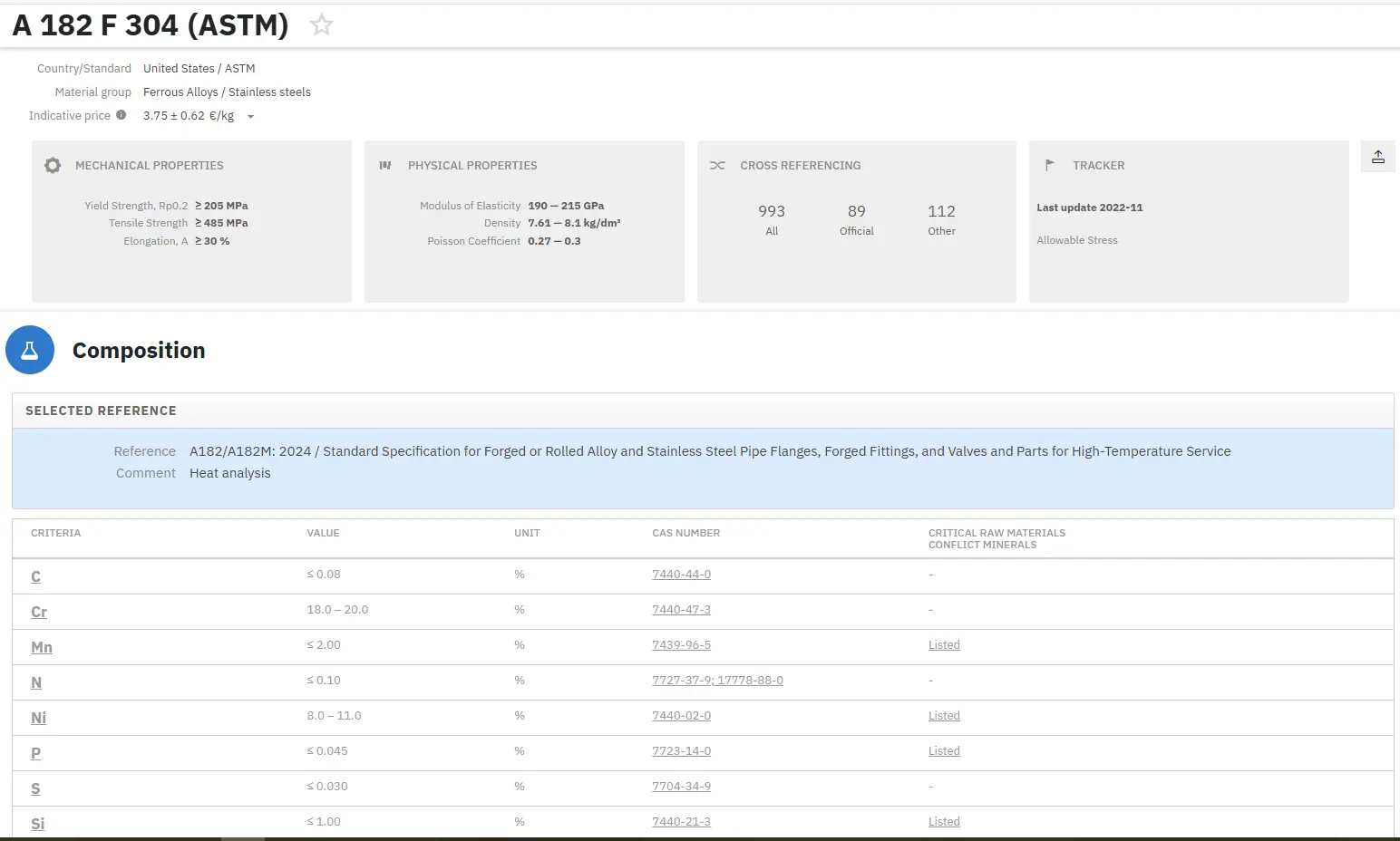
If the plant design engineers expect to do a good job of designing the plant and specifying material that will stand up to these irradiation effects, it is paramount that they understand in as much detail as possible how the nuclear radiation changes the properties of metals and alloys. They must also understand how much damage is done per unit of exposure of radiation and how this might change under varying conditions such as different temperatures or different chemical environments. The physical properties of a material that can be affected by nuclear radiation include density, elastic constants, stored energy, electrical resistivity, thermal conductivity, thermoelectric effect, and coefficient of thermal expansion. Mechanical properties that are affected by nuclear radiation include tensile strength, hardness, impact resistance, creep resistance, stress rupture failure, and fatigue.
The damage that leads to the effects on these various physical properties is initiated by interaction between energetic subatomic particles and the components atomic structure. In terms of damage producing capabilities the most important nuclear particles are fission fragments and fast neutrons. Fission fragments are only pertinent within the fuel material itself while neutrons, due to their wide energy spectrum and ability to travel relatively large distances, are pertinent to all the reactor structural materials. Other energetic subatomic particles such as electrons, protons, alpha particles, and gamma rays, can also initiate damage in various materials; however, their contribution to the total damage is negligible when compared with fission fragments and neutrons. In regard to structural materials, the design engineer is mainly interested in the effects of neutron radiation on its mechanical properties.
立即查看精确的化学成分!
Total Materia Horizon 包含数十万种材料和物质的化学成分,以及机械和物理性能等。
申请 Total Materia Horizon免费试用帐户,加入来自全球 120 多个国家超过 500,000 名用户的大家庭。