Nitrogen in Steels: Part One
Abstract
This comprehensive article examines the role and impact of nitrogen in steel manufacturing and properties. It explores nitrogen solubility in steel, various sources during steelmaking processes, and its behavioral characteristics during solidification. The study details nitrogen's effects on crucial steel properties including formability, hardness, strain aging, and impact properties. While nitrogen can enhance mechanical and corrosion properties when properly controlled, excessive amounts may lead to detrimental effects such as poor formability and reduced ductility. The article particularly emphasizes nitrogen's influence on welded materials and heat-affected zones. Understanding these relationships is crucial for optimizing steel properties and manufacturing processes.
Introduction to Nitrogen's Role in Steel
All steels contain nitrogen, which plays a crucial role in improving mechanical and corrosion properties when it remains in solid solution or precipitates as very fine and coherent nitrides. In austenitic steels, nitrogen addition simultaneously enhances fatigue life, strength, work hardening rate, wear resistance, and localized corrosion resistance.
High nitrogen martensitic stainless steels demonstrate superior resistance to localized corrosion (including pitting, crevice, and intergranular corrosion) compared to their carbon-containing counterparts. This improved performance has led to high nitrogen steels being recognized as a promising new class of engineering materials.
Nitrogen Solubility in Steel Manufacturing
The nitrogen solubility in steel follows specific patterns that can be expressed through several key equations:
½N2=[N] (1)
The equilibrium constant for nitrogen absorption is:
K=[ppmN] / (pN2)½ (2)
The reaction constant relates to free energy:
ΔG = ΔG0 + RT ln KN (3)
At equilibrium:
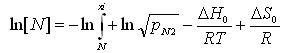 (4)
(4)
Assuming Henry's law applies, the nitrogen concentration can be calculated as:
[%N]=KN(pN2)½ (5)
Sources of Nitrogen in Steelmaking
Numerous sources of nitrogen exist during the melting, the ladle processing and the casting operations. Sources of nitrogen in oxygen steelmaking include the hot metal, the scrap, the impurity nitrogen in oxygen and nitrogen used as a stirring gas.
Nitrogen pickup from the atmosphere can occur during reblows in which case the furnace fills up with air, which is then entrained into the metal when the oxygen blow restarts. Also, during the tapping of steel, air bubbles are entrained into the steel where the tap stream enters the bath in the ladle. Other sources may include atmosphere (through ladle slag), coke (carburizers) and various ferro-alloys. Ladle additions often contain moisture. To get an impression of the sources of nitrogen during the melting process, Table 1 shows the amount of nitrogen present in each of the feed materials typically used in the EAF.
Table 1. Nitrogen content of feed materials used in EAF steelmaking
| Feed Material | Nitrogen Content |
| Scrap | 30-120 ppm |
| HBI/DRI | 20-30 ppm |
| Liquid iron from the BF | 60 ppm |
| Cold pig iron (CPI) | 20-30 ppm |
| Hot heel | 10 ppm |
| Coke | 5000-10000 ppm |
| Oxygen | 30-200 ppm |
| Carbon Carrier Gas (Air) | 78% |
| Bottom stirring gas(N2) | > 99,9% |
| Bottom stirring gas (Ar) | < 30 ppm |
| CaO | 400 ppm |
Nitrogen Behavior During Steel Processing
In liquid steel, nitrogen exists in solution. During solidification, three primary phenomena may occur:
- Blowhole formation
- Nitride compound precipitation
- Interstitial solid solution formation
The maximum nitrogen solubility in liquid iron reaches approximately 450 ppm, reducing to less than 10 ppm at ambient temperature. Dissolved sulfur and oxygen, being surface-active elements, limit nitrogen absorption - a characteristic utilized during steelmaking to prevent excessive nitrogen pickup.
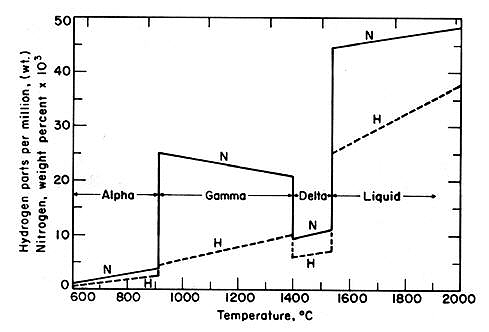
Figure 1: Solubility of nitrogen in iron for temperatures of 600-2000°C
Effects on Steel Properties and Performance
Effect of Nitrogen on Formability
In Low-Carbon Aluminum-Killed (LCAK) steels, nitrogen content significantly influences mechanical properties. As shown in Figure 2, steel strength initially decreases then increases with rising nitrogen levels. The elongation demonstrates an inverse relationship, decreasing as nitrogen content rises. The r-value, which represents the ratio of width to thickness strain in strip tensile specimens, increases with nitrogen content. This relationship indicates that higher nitrogen levels lead to reduced formability in LCAK steels, a characteristic that persists after annealing.
These mechanical property changes result from three key mechanisms: interstitial solid solution strengthening by free nitrogen, precipitation strengthening through aluminum and other nitrides, and grain refinement from nitride precipitates.
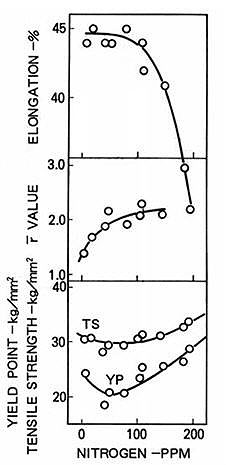
Figure 2: Effect of nitrogen on yield strength, tensile strength, r-value and elongation of LCAK steel
Effect of Nitrogen on Hardness
Surface indentation resistance, or hardness, increases linearly with nitrogen content. This relationship stems from nitrogen absorption during steelmaking, which promotes both interstitial solid solution strengthening and grain refinement. Nitrogen absorbed during the steelmaking process shows more pronounced effects compared to absorption during batch annealing in nitrogen-rich atmospheres, though both processes measurably affect final hardness values.
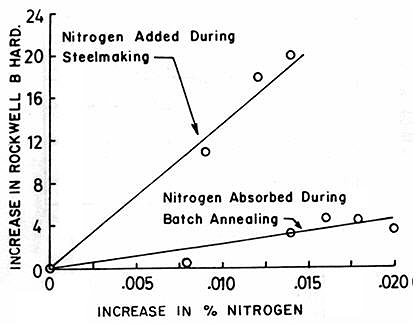
Figure 3: Increase in hardness of aluminum-killed sheet steel with respect to increasing nitrogen content
Effect of Nitrogen on Strain Ageing
Strain ageing manifests in steels containing interstitial atoms, particularly nitrogen, following plastic deformation. During this process, nitrogen migrates to dislocations, causing discontinuous yielding during subsequent deformation. This phenomenon increases hardness and strength while reducing ductility and toughness. Surface defects known as 'fluting' or 'stretcher strains' often appear as a result.
Duckworth and Baird developed the strain ageing index to measure this effect, calculating yield stress increases in deformed material held at room temperature for 10 days. Figure 4 demonstrates that higher nitrogen content correlates with increased strain-ageing index values, indicating greater susceptibility to surface defects.
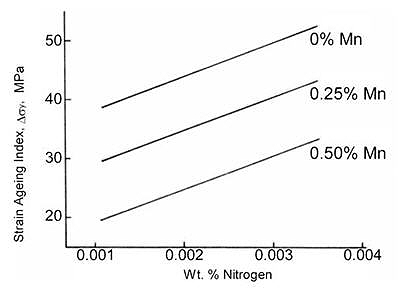
Figure 4: Effect of nitrogen on strain aging in mild steels
Effect of Nitrogen on Impact Properties
Material toughness, measured by energy absorption before fracture, varies significantly with nitrogen content. As temperature decreases, fracture behavior transitions from fibrous/ductile to crystalline/brittle. This transition temperature increases with higher free nitrogen content, resulting in decreased toughness through solid solution strengthening.
Interestingly, controlled amounts of nitrogen present as precipitates can enhance impact properties. Aluminum, vanadium, niobium, and titanium nitrides promote fine-grained ferrite formation. Smaller grain sizes lower the transition temperature, improving overall toughness. Therefore, optimizing impact properties requires careful control of both nitrogen content and its form within the steel.
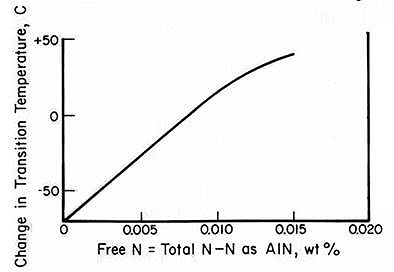
Figure 5: Effect of free nitrogen on impact properties
Nitrogen Effects in Welded Zones
The heat-affected zone (HAZ) of welded steel presents particular challenges. During welding, elevated temperatures can dissociate nitrides in the HAZ, leading to increased grain size. Rapid cooling then produces low-toughness martensite or bainite containing high levels of free nitrogen, further compromising toughness.
This HAZ embrittlement occurs primarily due to the dissociation of nitrides present in the HAZ during the high-temperature welding cycle. The absence of precipitates results in larger grain diameters, while rapid cooling produces microstructures with reduced toughness. The combination of these factors, along with increased free nitrogen levels, significantly impacts the mechanical properties of the welded region.
To mitigate these effects, welding procedures can be modified through lower heat input and multiple welding passes. These techniques help prevent complete nitride dissociation and maintain more favorable microstructural characteristics in the HAZ.
Daha fazla bilgi edinin
Malzemelerin Doğru Kimyasal Bileşimlerine Anında Ulaşın!
Total Materia Horizon yüz binlerce malzeme'nin kimyasal bileşimlerine ve içerik maddelerine, mekanik ve fiziksel özelliklerine ve çok daha fazlasına ulaşabilirsiniz.

Total Materia Horizon'da ÜCRETSİZ bir test hesabı edinin ve 120'den fazla ülkeden 500.000'den fazla kullanıcıdan oluşan bir topluluğa sizde katılın.