Austenitic Steels: Understanding Structure, Composition, and Properties
Abstract
Austenitic steels represent a crucial class of stainless steels characterized by their face-centered cubic crystal structure retained at room temperature through strategic alloying. Key austenite-forming elements like nickel and manganese extend the γ-loop in the iron-carbon equilibrium diagram, enabling austenite stabilization. Chromium addition provides exceptional corrosion resistance through protective oxide film formation, making chromium-nickel austenitic steels indispensable in corrosive environments. These steels typically contain 18-30% chromium, 8-20% nickel, and 0.03-0.1% carbon. The Schaeffler diagram effectively predicts phase composition using chromium and nickel equivalents. Austenitic steels offer superior fabrication properties, avoiding ductile-brittle transitions common in ferritic steels, establishing them as essential construction materials for demanding applications.
Introduction to Austenitic Steels
Austenitic steels have emerged as one of the most important classes of stainless steels in modern engineering applications. These materials derive their name from their predominant microstructural phase, austenite, which is characterized by a face-centered cubic crystal structure. The ability to retain this structure at room temperature, either in stable or metastable conditions, distinguishes austenitic steels from other steel categories and provides them with unique mechanical and corrosion-resistant properties.
The development of austenitic steels represents a significant advancement in materials science, combining the corrosion resistance of chromium with the structural stability provided by austenite-forming elements. This combination has resulted in materials that excel in demanding environments where both mechanical performance and corrosion resistance are critical requirements.
Fundamental Alloying Elements and Their Effects
Austenite-Forming Elements
Several elements play crucial roles in extending the γ-loop in the iron-carbon equilibrium diagram, with nickel and manganese being the most prominent examples. When sufficient quantities of these alloying elements are added to steel, they enable the preservation of face-centered cubic austenite at room temperature. This retention can occur in either stable or metastable conditions, depending on the specific composition and thermal history of the material.
Chromium presents an interesting case when considered independently. When added alone to plain carbon steel, chromium tends to close the γ-loop and favor ferrite formation. However, when chromium is combined with nickel in steel compositions, it retards the kinetics of the γ → α transformation, making it easier to retain austenite at room temperature. This synergistic effect between chromium and nickel forms the foundation of modern austenitic stainless steel compositions.
Corrosion Resistance Through Chromium Addition
The presence of chromium dramatically improves the corrosion resistance of steel through the formation of a very thin, stable oxide film on the surface. This protective layer, primarily composed of chromium oxide, provides excellent resistance to various corrosive environments. Consequently, chromium-nickel austenitic steels have become the most widely used materials for applications involving corrosive conditions, both at room temperature and elevated temperatures.
Composition and Phase Relationships in Austenitic Steels
Standard Compositional Ranges
Simple austenitic steels typically contain carefully balanced proportions of key alloying elements. Chromium content usually ranges between 18 and 30%, while nickel content varies from 8 to 20%. Carbon content is generally maintained between 0.03 and 0.1% to optimize the balance between strength and corrosion resistance.
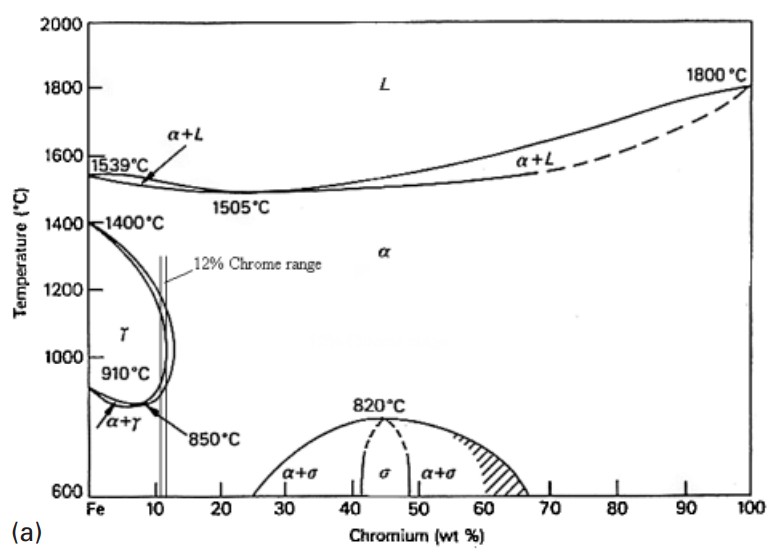
Figure 1: The Fe-Cr equilibrium diagram showing how chromium restricts the occurrence of the γ-loop
The binary iron-chromium equilibrium diagram illustrates how chromium restricts the occurrence of the γ-loop. Above 13% chromium, binary alloys remain ferritic over the entire temperature range, while a narrow (α + γ) range exists between 12% and 13% chromium. The ferrite phase in these compositions is typically referred to as delta ferrite, as it can exist continuously from the melting point to room temperature.
Effect of Carbon on Phase Relationships
Carbon addition to binary iron-chromium alloys significantly extends the γ-loop to higher chromium contents and widens the (α + γ) phase field up to 0.3% carbon content. When carbon is progressively added to an 18% chromium steel, distinct phase relationships emerge based on carbon content.
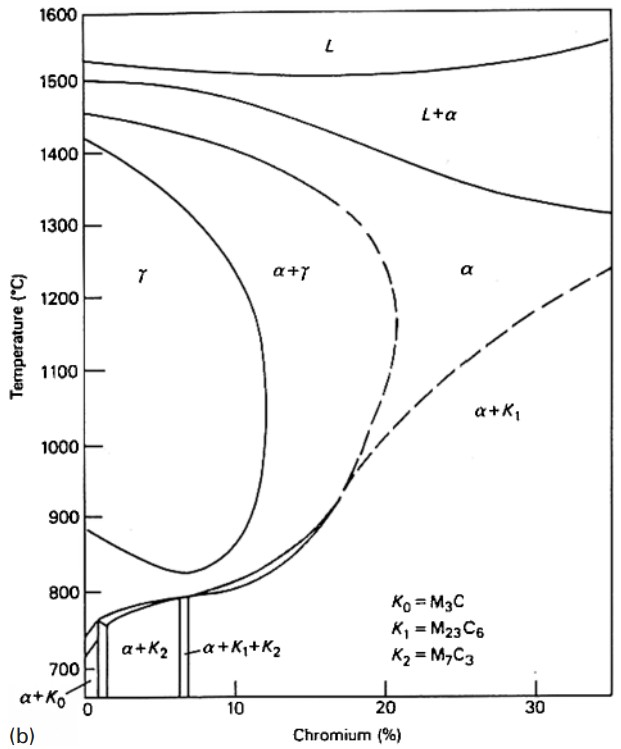
Figure 2: Effect of carbon on the Fe-Cr diagram demonstrating how carbon content influences phase relationships
In the range up to approximately 0.04% carbon, the steel remains fully ferritic and cannot be transformed. Between 0.08 and 0.22% carbon, partial transformation becomes possible, leading to (α + γ) structures. Above 0.40% carbon, the steel can be made fully austenitic if cooled rapidly from the γ-loop region.
Critical Composition Considerations
Nickel Requirements for Austenite Retention
The addition of nickel to low-carbon iron-18% chromium alloys expands the γ-phase field until, at approximately 8% nickel, the γ-phase persists to room temperature. This leads to the familiar group of austenitic steels based on the 18% chromium, 8% nickel composition. This particular composition represents a minimum nickel content required to retain austenite at room temperature.
The nickel requirement varies with chromium content. For more corrosion-resistant steels with higher chromium contents, such as 25% chromium compositions, approximately 15% nickel is needed to retain austenite at room temperature. The lack of complete austenite retention is indicated by martensite formation, with stable austenite defined as having an Ms temperature below room temperature.
Alternative Austenite-Forming Elements
Manganese serves as an alternative to nickel for austenite formation, though it is approximately half as effective. Higher concentrations are therefore required to achieve equivalent austenite stabilization. In the absence of chromium, around 12% manganese is required to stabilize high-carbon (1-1.2%) austenite, as achieved in Hadfield's steel. Typical chromium-manganese austenitic steels require 12-15% chromium and 12-15% manganese to remain austenitic at room temperature when carbon content is low.
Nitrogen represents another powerful austenite-forming element, functioning as an interstitial solute in austenite. Both carbon and nitrogen serve as the most effective solid solution strengtheners available. Nitrogen proves particularly useful as it has less tendency to cause intergranular corrosion compared to carbon. Nitrogen concentrations up to 0.25% can nearly double the proof stress of chromium-nickel austenitic steels.
The Schaeffler Diagram and Compositional Prediction
Understanding Phase Prediction
The Schaeffler diagram provides one of the most convenient methods for representing the effects of various elements on the basic structure of chromium-nickel stainless steels. This diagram, frequently used in welding applications, plots the compositional limits at room temperature for austenite, ferrite, and martensite phases in terms of nickel and chromium equivalents.
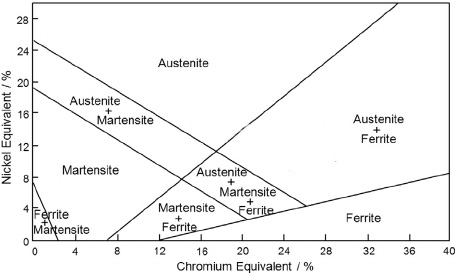
Figure 3: Schaeffler diagram showing the effect of alloying elements on the basic structure of Cr-Ni stainless steels
At its fundamental level, the diagram shows the regions of existence for the three phases in iron-chromium-nickel alloys. However, the diagram becomes much more broadly applicable when chromium and nickel equivalents are used to account for other alloying elements.
Equivalent Calculations
The chromium equivalent has been empirically determined using the most common ferrite-forming elements according to the following relationship:
Cr equivalent = (Cr) + 2(Si) + 1.5(Mo) + 5(V) + 5.5(Al) + 1.75(Nb) + 1.5(Ti) + 0.75(W)
Similarly, the nickel equivalent has been determined with familiar austenite-forming elements:
Ni equivalent = (Ni) + (Co) + 0.5(Mn) + 0.3(Cu) + 25(N) + 30(C)
All concentrations are expressed in weight percentages. The large influence of carbon and nitrogen relative to metallic elements should be particularly noted, as these interstitial elements have disproportionately strong effects on phase stability.
Practical Applications and Significance
Fabrication and Mechanical Properties
Austenitic steels offer significant advantages in fabrication and mechanical performance. These materials are readily fabricated and do not undergo the ductile-brittle transition that causes numerous problems in ferritic steels. This characteristic has ensured that austenitic steels have become an important group of construction steels, often specified for very demanding environments.
The absence of a ductile-brittle transition means that austenitic steels maintain their toughness and ductility across a wide temperature range, making them suitable for applications involving temperature variations or low-temperature service conditions.
Carbide Formation and Corrosion Considerations
In austenitic steels, M23C6 represents the most significant carbide formed, and it can substantially influence corrosion resistance. The formation and distribution of these carbides can affect the material's performance in corrosive environments, particularly regarding intergranular corrosion susceptibility.
The Schaeffler diagram proves particularly useful in determining whether a particular steel composition will be fully austenitic at room temperature. This application is relevant not only to bulk steels but also to weld metal, where predicting the microstructure is frequently important to avoid weld defects and excessive localized corrosive attack.
Conclusion
Austenitic steels represent a sophisticated class of materials that combine excellent corrosion resistance with superior mechanical properties and fabrication characteristics. The careful balance of alloying elements, particularly chromium for corrosion resistance and nickel for austenite stabilization, has created materials that serve critical roles in modern engineering applications. Understanding the phase relationships and compositional effects described by tools like the Schaeffler diagram enables engineers to select and specify appropriate austenitic steel compositions for specific service requirements, ensuring optimal performance in demanding environments.
Paslanmaz Çeliklerin Kesin Özelliklerine Hızlı Erişim!
Total Materia Horizon 120.000'den fazla paslanmaz çelik için özellik bilgileri içerir: kimyasal bileşim, mekanik ve fiziksel özellikler, doğrusal olmayan özellikler ve çok daha fazlası.
Total Materia Horizon'da ÜCRETSİZ bir test hesabı edinin ve 120'den fazla ülkeden 500.000'den fazla kullanıcıdan oluşan bir topluluğa sizde katılın.