Zn-Mg Alloys: Advanced Biocompatible Materials for Medical Implant Applications
Abstract
Zn-Mg alloys represent promising biocompatible materials for medical industry applications, offering superior mechanical strength and corrosion resistance for implant use. These alloys have gained significant attention as anticorrosion coatings for steels and biodegradable materials suitable for human body applications. Research demonstrates that Zn-Mg coatings with magnesium content below 10 wt.% exhibit substantially improved corrosion properties compared to pure zinc coatings. With superior mechanical properties compared to polymers, these metals provide an ideal platform for interim implants when biocompatibility matches or exceeds that of biodegradable polymers. Studies utilizing mechanical alloying and casting methods reveal that optimal magnesium content enhances microhardness and electrochemical properties, with ZnMg(3) alloy demonstrating exceptional corrosion resistance through nano-structured formations and protective surface layers.
Introduction to Zn-Mg Alloys in Medical Applications
Zn-Mg alloys present numerous solutions for addressing the critical need for suitable biocompatible materials in the medical industry. These alloys offer enhanced mechanical strength and corrosion resistance, making them ideal candidates for implant applications. As members of the light metal system, Zn-Mg alloys have attracted extensive attention over recent decades, particularly as anticorrosion coatings for steels and biodegradable materials designed for human body applications.
Recent research has demonstrated that Zn-Mg coatings containing magnesium content lower than 10 wt.% exhibit significantly improved corrosion properties compared to pure zinc coatings. This advancement positions these materials as potential candidates for next-generation galvanized steels. The superior mechanical properties of these metals, when compared to polymers, provide a more ideal platform for interim implants, particularly when their biocompatibility approaches or exceeds that of biodegradable polymers.
Mechanical Alloying Process and Sample Preparation
The study conducted by L. F. Guleryuz, R. Ipek, I. Arıtman, and S. Karaoglu focused on developing Zn-Mg alloys with high mechanical strength and corrosion resistance for implant applications. The research team processed Zn-Mg alloys using the mechanical alloying method, followed by hot sintering at 410°C under an argon atmosphere.
For sample preparation, the researchers utilized atomized magnesium and zinc powders with average grain sizes of approximately 63 μm and 99% purity. The mixture consisted of 20 wt.% magnesium content and 80 wt.% zinc content, prepared using a V-blender with one hour of mixing time. For comparison purposes, the same percentage Zn-Mg alloy underwent mechanical alloying using planetary ball milling with 10 mm-diameter stainless steel balls at a weight ratio of 10:1 and rotation speed of 250 r/min for eight hours.
The sintering process employed a high-strength graphite die measuring 10 mm in inner diameter and 60 mm in height. The ball-milled powders were placed into the graphite die and hot press sintered under 30 MPa pressure in an argon atmosphere. The heating process involved raising the temperature to 410°C at a rate of 50°C/min and maintaining this temperature for 30 minutes to achieve material homogenization.
Microstructural Analysis and Hardness Testing Results
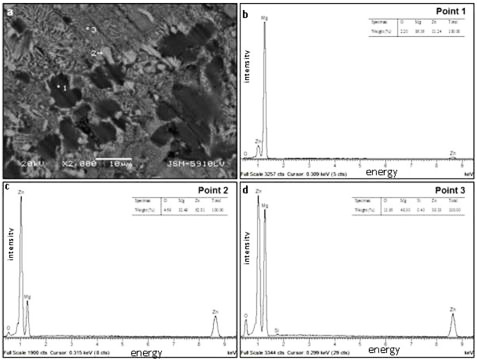
Figure 1: Sample energy dispersive spectrograph
The microstructural analysis revealed important insights into the alloy composition and behavior. Figure 1b presents the EDS results of point one, where the peak was identified as magnesium. Figure 1c displays the EDS results of point two, showing peaks identified as magnesium, zinc, and oxygen. Figure 1d presents point three, which occurred due to the chemical activity between the matrix and reinforcement compound during the sintering process.
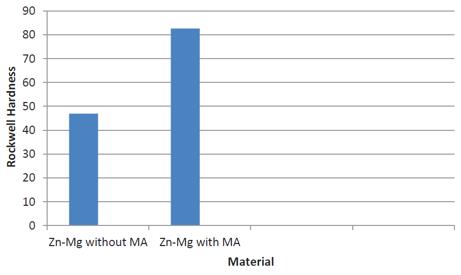
Figure 2: Rockwell hardness values after hot sintering process
Hardness measurements were conducted on polished samples using a Rockwell hardness tester machine, specifically a Matsuzawa mark DXT model hardness tester with a 15 kg load applied for 15 seconds. Each hardness value represented the average of five measurements, as illustrated in Figure 2.
Electrochemical Properties and Corrosion Resistance Investigation
The comprehensive study by C. Yao, Z. Wang, S. Leng Tay, T. Zhu, and W. Gao investigated the effect of magnesium addition on the microstructure and electrochemical properties of Zn-Mg alloys. Their research explored the mechanism behind the improved corrosion resistance of Zn-Mg alloys.
The research team prepared Zn-Mg alloys with varying magnesium contents using a casting method, producing approximately 250 g cast blocks for each composition. High-purity zinc metal (>99.90%) was melted in an MgO crucible using a molten salt furnace at 560°C. MgZn2 intermetallic was then added to the molten zinc under shielding argon gas to prevent magnesium oxidation. The melt underwent homogenization through intense mechanical stirring before being poured into a permanent mold and cooled under argon atmosphere protection.
Table 1. Checmical composition of the investigated alloys
| Symbol | Nominal composition | Zn | Mg |
| Zn | Zn | 100 | 0 |
| ZnMg(1) | Zn-1 wt%Mg | 99.40 | 0.60 |
| ZnMg(2) | 97.68 | 2.32 | |
| ZnMg(3) | 96.88 | 3.12 | |
| ZnMg(5) | 95.44 | 4.56 | |
| MgZn2 | 84.56 | 15.44 |
The obtained Zn-Mg alloys were cut into 20 mm x 20 mm x 2 mm samples for testing. The chemical compositions, as determined by EDS analysis, are presented in Table 1. The measured compositions differed from nominal values, likely due to magnesium oxidation or metal residuals adhering to the mechanical mixer or crucible.
Advanced Characterization Techniques and Testing Methods
The microstructural analysis employed sophisticated equipment including optical microscopy (Olympus BX60 M) and environmental scanning electron microscopy (ESEM, Philips XL-30S) equipped with energy dispersive spectroscopy (EDS, SUTW-Sapphire). Metallurgical specimens underwent grinding and polishing to 1 μm following standard procedures, followed by etching using 5 wt.% Nital for 20 seconds to reveal microstructural details.
XRD phase analysis was conducted using a Philips PW 1710 system (U = 40 kV, I = 40 mA) with Cu Kα irradiation (λ = 0.15406 nm). Microhardness measurements utilized a Leco M400 tester with a Vickers diamond indenter, applying a 100 g load with a 15-second holding time. At least four measurements were conducted on each specimen under identical conditions, with average values used as the microhardness (HV) results.
Electrochemical Testing and Performance Evaluation
The electrochemical properties of Zn-Mg alloys in 3.5 wt.% NaCl solution were investigated using a CHI604D electrochemical workstation at 20°C. The testing setup employed a flat cell configuration with the Zn-Mg alloy as the working electrode, a platinum sheet as the counter electrode, and an Ag/AgCl electrode in saturated KCl as the reference electrode.
Specimens underwent grinding and polishing to 1 μm, followed by ethanol cleaning before electrochemical testing. A surface area of 1 cm² was exposed to the saline solution during testing. The testing protocol involved initial immersion to achieve stable open circuit potential (OCP), followed by electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization curve measurements.
Research Conclusions and Performance Insights
The comprehensive research yielded several significant conclusions regarding Zn-Mg alloy performance. The microhardness of specimens increased proportionally with magnesium content, ranging from 43.40 HV for pure zinc to 345.75 HV for MgZn2. The ZnMg(3) alloy demonstrated nano-structure formation and exhibited the best corrosion resistance among tested compositions.
Potentiodynamic polarization curves revealed that the Ecorr of ZnMg(3) alloy was more positive than pure zinc, with a low icorr approximately 34% of zinc's value. However, further increases in magnesium content resulted in decreased corrosion resistance, indicating an optimal composition range.
EIS testing provided additional insights through fitting equivalent circuit analysis, simulating the corrosion process of ZnMg(3). The impedance of ZnMg(3) alloy significantly exceeded that of pure zinc across the frequency range of 1 to 100,000 Hz, with the alloy exhibiting a wave crest close to 53°.
Future Applications and Material Advantages
The nano-structured ZnMg(3) alloy demonstrates a tendency to form uniform protective layers of magnesium-containing corrosion products on the surface. This characteristic contributes to the general precipitation of magnesium-modified simonkolleite and effectively retards localized corrosion. These properties position Zn-Mg alloys as promising candidates for advanced biomedical applications, particularly in temporary implant systems where controlled degradation and biocompatibility are essential requirements.
The research findings support the continued development of Zn-Mg alloys for medical applications, with particular emphasis on optimizing magnesium content to achieve the ideal balance between mechanical properties and corrosion resistance. These materials represent a significant advancement in biodegradable metal technology for medical implant applications.
Мгновенный доступ к тысячам металлографических изображений!
Total Materia Horizon содержит уникальную коллекцию металлографических изображений различных сплавов, стандартов и термических обработок.
Получите бесплатный тестовый аккаунт в Total Materia Horizon и присоединяйтесь к сообществу из более чем 500 000 пользователей из 120+ стран.