Extractive Metallurgy of Non-Ferrous Metals: Part One
Abstract
Extractive metallurgy represents one of the most fundamental and critical areas of metallurgical science, focusing on the extraction of metals from ores, concentrates, scrap materials, and other sources for subsequent refining to liquid or solid states. This field encompasses three major processing methods: pyrometallurgy, hydrometallurgy, and electrometallurgy. As the art and science of metal extraction and refinement, extractive metallurgy remains essential to both developed and developing economies worldwide, providing crucial resources for metallic, mechanical, electromagnetic, electrical, and electronics industries. The production of metals and alloys through these sophisticated processes continues to serve as a cornerstone industry supporting global technological advancement.
Introduction to Extractive Metallurgy
Extractive metallurgy encompasses the comprehensive process of metal extraction from ores, concentrates (enriched ores), scraps, and various other sources, followed by their refinement to achieve either liquid metal states suitable for casting or refined solid metals. The extraction and refining operations required for these transformations involve various sophisticated metallurgical reaction processes that have been developed and refined over centuries of industrial advancement.
The outputs and products generated through extractive metallurgy serve as essential resources for numerous industries, including metallic manufacturing, mechanical engineering, electromagnetic applications, electrical systems, and electronics production. For these industrial purposes, silicon is also treated as a metal, expanding the scope of extractive metallurgy applications.
Classification of Extractive Metallurgy Methods
Traditionally, extraction and refining methods have been systematically classified into three primary categories, each offering distinct advantages and applications depending on the specific materials and desired outcomes.
Pyrometallurgy: High-Temperature Processing
Pyrometallurgical processes, derived from the Greek word 'pyr' meaning 'fire,' are conducted at elevated temperatures. These high-temperature operations can be further subdivided into two distinct processing categories:
1. Solid-State Processing operates without involving any melting phases and typically occurs within the temperature range of 500-1200°C. This method includes processes such as sulfide roasting, calcination, and solid-state reduction of metal oxides using hydrogen and calcium oxide. Since solids remain largely immiscible during these processes, the resulting products are either pure metals or mechanical mixtures requiring additional separation procedures.
2. Liquid-State Processing involves melting at least the metal-containing phase and generally operates at higher temperatures than solid-state methods. Notable examples include blast furnace smelting, steelmaking operations, and distillation refining of zinc from impure lead. Liquid-state processing effectively separates metals in either pure or impure forms, with significant compositional changes possible due to enhanced miscibility, rapid diffusion, and thorough mixing capabilities.
Hydrometallurgy: Aqueous Solution Processing
Hydrometallurgy, derived from the Greek word 'hydor' meaning 'water,' utilizes aqueous media for metal extraction and processing at or near room temperature. This method offers environmental advantages and precise control over chemical reactions.
Electrometallurgy: Electrolytic Separation
Electrometallurgy employs electrolysis techniques for metal separation, operating effectively at both room temperature and elevated temperatures depending on specific application requirements.
The Mineral Processing Chain
Raw ores cannot be directly utilized as finished products for industrial or commercial applications. The transformation chain leading to final metal production represents a technically coherent sequence of processes, including essential physical treatments such as grinding and flotation processes.
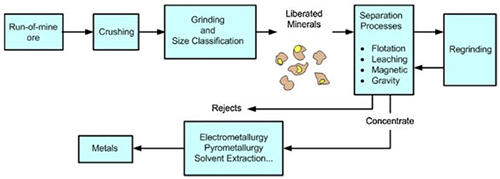
Figure 1: Process flow chain of non-ferrous extractive metallurgy
Mineral Processing Fundamentals
Mineral processing involves utilizing physical processes to manipulate ore particle size and concentrate valuable minerals through separation techniques. These separation methods rely on various ore properties, including density, chemical composition, electrostatic characteristics, magnetic properties, and fluorescence behaviors. Froth flotation serves as an excellent example of an effective separation process widely employed in the industry.
Additionally, mineral processing encompasses the separation of mineral solids from water and aqueous solutions through thickening, filtering, and drying operations, ensuring optimal material preparation for subsequent metallurgical processes.
Pyrometallurgical Operations
Pyrometallurgy involves treating ores at high temperatures to convert ore minerals into raw metals or intermediate compounds suitable for further refining. The most common pyrometallurgical processes include roasting, smelting, and converting operations.
Roasting Processes
Roasting processes extract metals from sulfide ores by heating the ore in oxygen-rich environments. During this process, sulfur undergoes oxidation and is expelled as sulfur dioxide gas. Some metals retain their sulfide form throughout the process, while others transform into oxide forms. The desired metal may be present in either resulting product, depending on specific process conditions and ore characteristics.
Smelting and Converting Operations
Oxidative smelting and converting operations share similarities with roasting processes but operate at temperatures sufficiently high to promote material melting. Certain minerals demonstrate resistance to oxidation and maintain their sulfide forms, while other minerals undergo complete oxidation and form compounds with additives, commonly referred to as flux materials. The resulting molten sulfides and oxide compounds separate into distinct layers due to their different specific weights.
However, these operations produce sulfur dioxide and carbon dioxide as byproducts, which represent significant environmental pollutants requiring careful management and treatment.
Hydrometallurgical Processes
Hydrometallurgy utilizes aqueous solutions to extract metals or compounds from their respective ores through various specialized processes. Key hydrometallurgical processes include leaching, precipitation of insoluble compounds, and pressure reduction techniques.
Leaching Operations
Leaching represents a chemical dissolution process for extracting desired minerals using aqueous solutions. The varying dissolution rates of different materials enable effective separation of compounds containing different metals. Frequently, oxidative reagents must be added to promote and enhance the leaching process, ensuring optimal metal recovery rates and processing efficiency.
This comprehensive approach to extractive metallurgy continues to evolve with technological advances, environmental considerations, and increasing demands for sustainable metal production methods. The integration of these various processing techniques enables the efficient extraction and refinement of non-ferrous metals essential for modern industrial applications.
Мгновенный доступ к точному химическому составу материалов!
Total Materia Horizon содержит химические составы сотен тысяч материалов и веществ, а также их механические и физические свойства и многое другое.

Получите бесплатный тестовый аккаунт в Total Materia Horizon и присоединяйтесь к сообществу из более чем 500 000 пользователей из 120+ стран.