Extractive Metallurgy of Non-Ferrous Metals: Part One
Abstract
One of the most fundamental yet critical areas of metallurgy relates to the extracting of metals from ores, concentrates, scrap and other sources with a view to refining to the liquid state.
Three methods can be considered as the major processes for carrying our extractive metallurgy activities, pyrometallurgy, hydrometallurgy, and electrometallurgy.
Extractive metallurgy is the art and science of extracting metals from their ores and refining them. The production of metals and alloys from these source materials is still one of the most important and fundamental industries in both developed and developing economies around the world. The outputs and products are essential resources for the metallic, mechanical, electromagnetic, electrical and electronics industries (silicon is treated as a metal for these purposes).
Generally speaking, extractive metallurgy is the process of the extraction of metals from ores, concentrates (enriched ores), scraps, and other sources and their refining to the state of either liquid metal before casting or to solid metals. The extraction and refining operations that are required may be carried out by various metallurgical reaction processes.
Traditionally, methods of extraction and refining have been classified into the following categories:
- Pyrometallurgy
- Hydrometallurgy
- Electrometallurgy
Pyrometallurgical processes (in Greak, ‘pyr’ means ‘more at fire’) are carried out at high temperatures. Hydrometallurgy (in Greak, ‘hydor’ means ‘more at water’) is carried out in aqueous media at or around room tempearture. Electrometallurgy employs electrolysis for separation at room temperature as well as at high temperature.
Another method of classification can be in terms of unit operation or unit process. Pyrometallurgy can be further classified as follows:
1. Solid-state processing: This does not involve any melting. It is typically carried out in the temperature range of 500-1200°C, as exemplified by the roasting of sulphides, calcination, solid-state reduction of metal oxides by H2 and Cao. Solids are mostly immiscible and hence the product of solid state processing is either pure or is a mechanical mixture. In the latter case, it requires further separation.
2. Liquid-state processing: This involves melting of at least the metal-containing phase and is on the whole carried out at a higher temperature. Examples are blast furnace smelting, steelmaking, distillation refining of zinc from impure lead etc. Liquid state processing separates out the metal either in pure or in impure form. Appreciable compositional changes in the liquid are possible due to miscibility, rapid diffusion and mixing.
As mentioned above, a raw ore cannot be used as such as a finished product for industrial or commercial uses.
The steps of the transformation chain, which lead to the production of the final metal, is a technically coherent sequence of processes, which include physical treatments of the ore (grinding and flotation processes).
Figure 1 shows the simplified chain of processes in mineral processing and metallurgical plants:
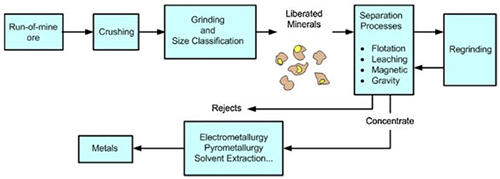
Figure 1: Process flow chain of non-ferrous extractive metallurgy
Mineral processing
Mineral processing involves the use of physical processes to manipulate ore particle size, and concentrate valuable minerals using the processes of separation, based on such properties of the ore, such as density, chemical composition, electrostatic, magnetic or fluorescence properties. A good example of a separation process is froth flotation.
Also of interest to the mineral processor is the separation of mineral solids from water and aqueous solutions by thickening, filtering and drying.
Pyrometallurgy
Pyrometallurgy involves the treatment of ores at high temperature to convert ore minerals to raw metals, or intermediate compounds for further refining. Roasting, smelting and converting are the most common pyrometallurgical processes.
A roasting process is used to extract metals from sulfide ores: in this process the ore is heated in the presence of oxygen and the sulfur is oxidized and driven off as sulfur dioxide. Some metals in this process remain in the sulfide form, while other metals are turned into an oxide form. The desired metal may be in either product.
Oxidative smelting and converting are similar to the roasting process, but differ slightly in the way that the processes' temperatures are high enough to promote melting of materials. Some minerals are more reluctant to oxidation, so they remain in the sulfide form, while other minerals are completely oxidized and form compounds with additives, often called flux. Molten sulfides and oxide compounds split in two layers because of the different specific weights.
The subsequent bi-products of these operations, sulfur dioxide and carbon dioxide, are major pollutants.
Hydrometallurgy
Hydrometallurgy involves the use of aqueous solutions to extract metals or compounds from their ores. Some of the hydrometallurgical processes include leaching, precipitation of insoluble compounds, pressure reduction.
Leaching is a process for chemical dissolution of the desired minerals in aqueous solutions. Due to the difference in the dissolution rates, it is possible to separate the compounds of different metals. Often, some oxidative reagents need to be added to promote leaching.
Seguir leyendo
¡Encuentre al Instante la Composición Precisa de los Materiales!
Total Materia Horizon contiene la composición química de cientos de miles de materiales y sustancias, así como sus propiedades mecánicas y físicas, y mucho más.
Obtenga una cuenta de prueba GRATUITA de Total Materia Horizon y únase a nuestra comunidad que traspasa los 500.000 usuarios provenientes de más de 120 países.