Corrosion and Corrosion Properties of Stainless Steels: Part Two
Abstract
Stainless steels, like all metals relying on passive films for corrosion resistance, are susceptible to localized corrosion including pitting and crevice corrosion. These corrosion types typically occur in chloride-containing aqueous solutions such as seawater and environments with other halogenides. Pitting creates holes in metal surfaces through extremely localized attack, while crevice corrosion develops in narrow, solution-containing spaces where passive films weaken. Chromium, molybdenum, and nitrogen enhance resistance to both corrosion types. The Pitting Resistance Equivalent (PRE) formula effectively combines these alloying elements' effects. Resistance to localized corrosion in seawater requires 6% molybdenum or more. Proper material selection and equipment design can significantly minimize localized corrosion risks in stainless steel applications.
Understanding Pitting and Crevice Corrosion in Stainless Steels
Stainless steels rely on passive films for corrosion resistance, making them susceptible to localized corrosion attacks. The protective passive film contains microscopic defects that typically do not compromise corrosion resistance. However, when halogenides such as chlorides are present in the environment, they can locally break down the passive film and prevent reformation of new protective layers. This breakdown leads to localized corrosion, specifically pitting or crevice corrosion. Both corrosion types commonly occur in chloride-containing aqueous solutions such as seawater, though they can also develop in environments containing other halogenides.
Characteristics and Mechanisms of Pitting Corrosion
Pitting represents an extremely localized form of attack that creates holes in metal surfaces. These holes vary in diameter but are typically relatively small, with surface diameters equal to or much less than their depth. Pitting corrosion exhibits particularly insidious characteristics, requiring extended initiation periods ranging from months to years before visible pits appear. The duration depends on both the specific metal composition and the corrosive environment conditions.
In highly aggressive environments such as acid media containing ferric chloride, serious pitting of stainless steels like X2CrNi19-11 (304L) can occur within days. Once initiated, pits penetrate metal at ever-increasing rates and tend to undermine or undercut surfaces as they grow. The diameter of pits can expand extensively below the surface, and pits typically grow in the direction of gravity. Most pits develop and grow downward from horizontal surfaces, with fewer starting on vertical surfaces and rarely growing upward from horizontal surface bottoms.

Figure 1: Pitting occurrence depending on Mo content in AISI 304
Crevice Corrosion Development and Prevention
Crevice corrosion occurs in narrow, solution-containing crevices where passive films are more readily weakened and destroyed. Common locations include areas under washers, flanges, deposits, or fouling on steel surfaces. This corrosion type results from differences in oxygen or metal ion concentration between crevices and their surroundings. Local differences in solution composition cause potential differences on immersed metals, accelerating corrosion processes. Differential aeration, involving differences in oxygen content, is particularly important in this context.
Crevice corrosion typically stems from one or more factors: lack of oxygen in crevices, build-up of detrimental ions, changes in acidity (decreased pH), or depletion of inhibitors within crevices.
Effective preventive measures include applying more resistant construction materials such as high nickel molybdenum alloys, making full penetration welds to avoid crevices, designing vessels for complete drainage, welding instead of rolling tubes in tube sheets, inspecting vessels and removing deposits frequently, removing wet packing materials during long shutdowns, and using solid non-absorbent gaskets such as PTFE.

Figure 2: Crevice corrosion under a rubber washer in a flat heat exchanger used in brackish water
Alloying Elements and Resistance Enhancement
Both corrosion forms occur in neutral environments, though attack risk increases in acidic solutions. Chromium, molybdenum, and nitrogen are the primary alloying elements that increase stainless steel resistance to both pitting and crevice corrosion. Resistance to localized corrosion in seawater requires 6% molybdenum or more.
Pitting Resistance Equivalent (PRE) Calculations
The Pitting Resistance Equivalent (PRE) effectively combines alloying element effects, accounting for different impacts of chromium, molybdenum, and nitrogen. Several equations exist for PRE calculations, each with slightly different coefficients for molybdenum and nitrogen. The most commonly used formula is:
PRE = %Cr + 3.3 x %Mo + 16 x %N
This formula is almost always applied to duplex steels and sometimes to austenitic steels. For austenitic steels, the nitrogen coefficient is often set to 30, while other coefficients remain unchanged:
PRE = %Cr + 3.3 x %Mo + 30 x %N
The difference between formulas is generally small, but the higher nitrogen coefficient creates differences in PRE values for nitrogen-alloyed grades.
Table 1. Typical PRE values for various stainless steels
| Grade | 304L | 316L | 'SAF 2304' | 317L | '2205' | '904L' | 'SAF 2507' | '254 SMO' | '654 SMO' |
| PRE16xN | 19 | 26 | 26 | 30 | 35 | 36 | 43 | 43 | 56 |
| PRE30xN | 20 | 26 | 30 | 37 | 46 | 63 |
Critical Temperature Testing and Correlation Analysis
Composition effects can be illustrated by plotting critical pitting temperature (CPT) in specific environments against PRE values for various steel grades. The relationship between critical pitting temperature in 1M NaCl and PRE values demonstrates how CPT values represent the lowest temperatures at which pitting corrosion attack occurred during testing.
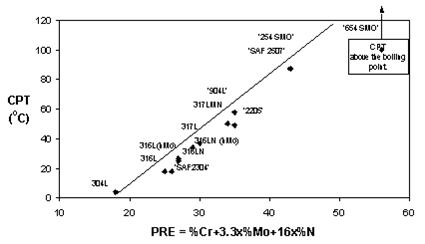
Figure 3: Pitting temperature (CPT) in 1M NaCl as a function of PRE-values
Since basic corrosion mechanisms are identical for both pitting and crevice corrosion, the same elements benefit both corrosion attack types. This similarity creates direct correlations between CPT and CCT values for specific steel grades.
Crevice corrosion is more severe than pitting corrosion, resulting in lower CCT values compared to CPT values for any stainless steel grade. This relationship is demonstrated when critical pitting temperature (CPT) and critical crevice corrosion temperature (CCT) in 6% FeCl3 are plotted against PRE values for various stainless steels.
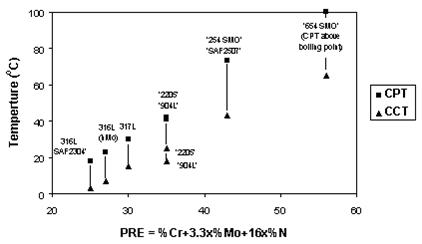
Figure 4: Critical pitting temperature (CPT) and critical crevice corrosion temperature (CCT) in ferric chloride for various stainless steels
Practical Applications and Limitations of PRE Values
The diagrams demonstrate relatively good correlation between PRE values and CPT and CCT measurements. Consequently, PRE values can group steel grades and alloys into rough categories with similar localized corrosion attack resistance, typically in steps of 10 PRE units. However, PRE values cannot effectively compare or separate steel grades or alloys with nearly similar PRE values.
All diagrams of this type show comparisons between steel grades and are only valid for given test environments. Steel grades exhibit different CPT values in NaCl compared to FeCl3 environments. Therefore, temperatures in diagrams cannot be applied to other environments unless practical experience demonstrates relationships between actual service conditions and testing conditions.
Relative ranking of localized corrosion resistance often remains consistent across different environments. The closer test environments simulate principal factors of service environments, the more reliable the generated data becomes for judging steel grade suitability for specific service environments. Sodium chloride testing is consequently superior to ferric chloride testing for evaluating grade suitability in neutral pH, chloride-containing water solutions common in many industries.
Design Considerations for Corrosion Prevention
Obtaining good resistance to both pitting and crevice corrosion requires selecting highly alloyed stainless steels with sufficiently high molybdenum content. However, choosing appropriate grades is not the only method for minimizing localized corrosion attack risks. Design-stage risk reduction involves avoiding stagnant conditions and narrow crevices. Designers can minimize pitting and crevice corrosion risks through both correct steel grade selection and appropriate equipment design.
Accedi subito a proprietà precise sulla corrosione!
Total Materia Horizon contiene informazioni sul comportamento alla corrosione e sulle proprietà di centinaia di migliaia di materiali, in oltre 2.000 ambienti.

Ottieni un account di prova GRATUITO su Total Materia Horizon e unisciti a una comunità di oltre 500.000 utenti provenienti da più di 120 paesi.