Atmospheric Corrosion of Aluminum Alloys
Abstract
Relative humidity, temperature, sunshine, pollutants, salinity all play a significant role in the atmospheric corrosion rate of materials and when very specific conditions are taken into consideration e.g. subtropical marine environments, can result in some of the most extreme corrosion conditions possible.
As one of the most widely produced metals on the planet, aluminum is the subject of many investigations in this area with at times, some interesting results.
Except for ferrous metals, aluminum is one of the most produced metals globally. Aluminum has many advantageous properties such as lightness, suitability for surface treatments, functional advantages of extruded and cast semi-products, high thermal and electrical conductivity. Aluminum forms a diverse group of alloys, which gives a wide range of properties and uses. It is also easy to form and recycle; the recycling of aluminum requires only 5% of the energy it takes to extract the metal from its ore. Aluminum has good corrosion resistance, especially in the atmosphere, due to the natural oxide layer.
Atmospheric corrosion is one of the most common types of corrosion responsible for the degradation metallic structures, devices, and products exposed to the atmosphere. Atmospheric conditions such as relative humidity, temperature, sunshine period’s pollutants, salinity, etc. play an essential role in corrosion of the exposed metals. In particular, the tropical and subtropical marine environment introduces one of the most aggressive conditions which results in serious atmospheric corrosion of metals.
Aluminum is widely used in plenty of industrial applications such as construction, electrical engineering, transport, and especially in the food industry for the manufacture of processing, production, storage and transportation equipment and machinery.
In the paper of Li T. et al., corrosion product formed on 2A12 Al alloy after 3 months of exposure in South China Sea atmosphere was analyzed by various surface analysis techniques, including scanning electron microscopy (SEM), energy-dispersive x-ray analysis (EDXA), x-ray protoelectron spectroscopy (XPS), and x-ray diffraction (XRD). The mechanism for atmospheric corrosion of Al alloy in the tropical marine environment was analyzed too. The authors realized that the atmospheric exposure usually took 1 year or more for a study cycle. Furthermore, it is worth pointing out that there is no typical four-season climate in the test location.
Figure 1 shows the optical views of macroscopic morphology of the top and bottom of the Al alloy specimen after 3 months of atmospheric exposure. It is clear that the specimen was heavily corroded, with localized corrosion occurring on the specimen surface. Both views show that the specimen lost brightness and became rough, with a yellowish gray patina present on the surface. Moreover, corrosion of the Al alloy specimen on the bottom was more serious than that on the top section.
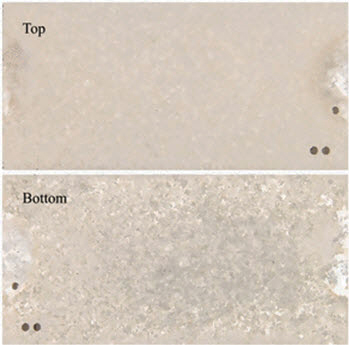
Figure 1: Optical views of surface morphology of top and bottom parts of Al alloy specimen after 3 months of exposure in Xi-Sha Island atmosphere
Accedi subito a proprietà precise sulla corrosione!
Total Materia Horizon contiene informazioni sul comportamento alla corrosione e sulle proprietà di centinaia di migliaia di materiali, in oltre 2.000 ambienti.

Ottieni un account di prova GRATUITO su Total Materia Horizon e unisciti a una comunità di oltre 500.000 utenti provenienti da più di 120 paesi.