Characteristics of Alloying Elements in Steel Manufacturing
Abstract
This comprehensive analysis examines the fundamental characteristics and applications of key alloying elements in steel production, including manganese, nickel, chromium, molybdenum, vanadium, tungsten, silicon, copper, cobalt, and boron. These elements significantly influence steel properties such as tensile strength, hardness, ductility, corrosion resistance, and heat treatment response. Manganese serves as a deoxidizer and improves ductility when combined with reduced carbon content. Nickel enhances strength and toughness while lowering transformation temperatures. Chromium provides exceptional hardness and wear resistance, particularly in tool steels and stainless applications. The synergistic effects of nickel-chromium combinations produce superior mechanical properties for structural applications. Modern steel alloy design increasingly focuses on optimizing element combinations to achieve specific performance characteristics while managing production costs and material availability.
Introduction to Steel Alloying Elements
The development of modern steel alloys relies heavily on understanding how specific alloying elements modify the fundamental properties of iron-carbon systems. Critical elements for advanced steel manufacturing include manganese, nickel, chromium, molybdenum, vanadium, tungsten, silicon, copper, cobalt, and boron. Each element contributes unique characteristics that enable engineers to design steels for specific applications ranging from automotive components to aerospace structures.
Commercial steel production universally incorporates manganese in concentrations of 0.3-0.8% to function as a deoxidizer and counteract the detrimental effects of iron sulfide. Contemporary metallurgical practices increasingly favor higher manganese content paired with reduced carbon levels to achieve equivalent tensile strength while significantly improving ductility and impact resistance.
Manganese: The Essential Deoxidizer and Strength Enhancer
Basic Properties and Functions
All commercial steel grades contain manganese concentrations between 0.3-0.8% primarily to reduce oxide inclusions and neutralize the harmful influence of iron sulfide. Manganese beyond these baseline requirements partially dissolves into the iron matrix while forming Mn₃C carbides that coexist with Fe₃C cementite structures.
Modern steel alloy design demonstrates a clear trend toward increased manganese content combined with reduced carbon concentrations. This approach maintains equivalent tensile strength levels while delivering substantially improved ductility characteristics. However, manganese concentrations exceeding 1.8% tend to promote air hardening behavior, which can compromise ductility properties.
Applications in Specialized Steel Grades
Manganese additions up to 1.8% provide beneficial effects on the mechanical properties of oil-hardened and tempered steels containing 0.4% carbon. Economic considerations drive the increased use of manganese in certain alloy systems, particularly as a partial replacement for expensive nickel. Steel compositions featuring 0.3-0.4% carbon, 1.3-1.6% manganese, and 0.3% molybdenum successfully replace 3% nickel steels in many structural applications.
Non-shrinking tool steels incorporate up to 2% manganese combined with 0.8-0.9% carbon content. Steels containing 5-12% manganese exhibit martensitic transformation after slow cooling but find limited commercial application due to processing challenges.
Hadfield Manganese Steel Applications
Hadfield's manganese steel represents a specialized high-manganese alloy containing 12-14% manganese and 1.0% carbon. This composition exhibits exceptional wear resistance, making it ideal for railway points, rock drilling equipment, and stone crushing machinery. The steel requires quenching from 1000°C to retain austenitic structure in its soft, workable condition.
A unique characteristic of Hadfield steel involves work hardening during abrasion service. Surface layers increase in hardness from 200 to 600 VPN without magnetic property changes, while underlying material maintains toughness. Annealing treatments prove detrimental as they promote carbide formation at grain boundaries, leading to embrittlement.
Nickel: Strength and Toughness Enhancement
Transformation Temperature Effects
Nickel and manganese exhibit similar behavioral patterns in steel alloys, both effectively lowering eutectoid temperatures. Increasing nickel content progressively reduces heating transformation temperatures by approximately 10°C per 1% nickel addition. However, cooling transformation temperature reductions prove greater and more irregular than heating effects.
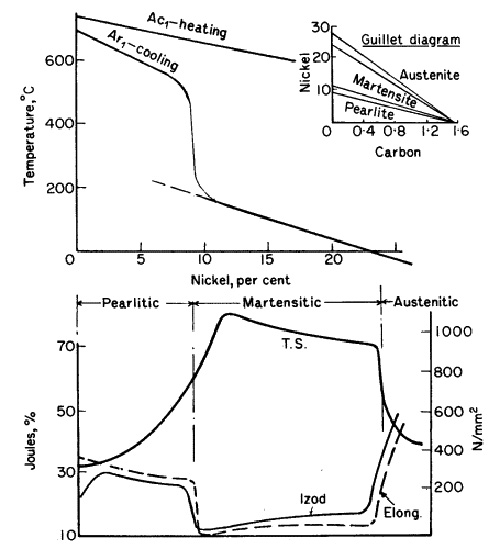
Figure 1: Effect of nickel on transformation temperatures and mechanical properties
The transformation temperature curve for different nickel contents in 0.2% carbon steels shows a dramatic decrease around 8% nickel content. Steels containing 12% nickel begin transformation below 300°C during cooling, but reverse transformation during heating requires temperatures around 650°C. These characteristics define irreversible steels with pronounced hysteresis effects, utilized in maraging steels and 9% Ni cryogenic applications.
Microstructural Development
Nickel additions produce effects similar to increased cooling rates in carbon steels. With constant cooling rates, 5-8% nickel steels develop troostitic structures, while 8-10% nickel compositions where the transformation temperature drops sharply produce martensitic structures. Nickel contents above 24% depress critical transformation temperatures below room temperature, resulting in retained austenitic structures.
Mechanical properties change correspondingly with microstructural development. Steels with 0.5% nickel resemble carbon steels but demonstrate superior strength due to finer pearlite formation and nickel solution strengthening in ferrite. Nickel contents exceeding 10% produce high tensile strength and hardness but suffer from brittleness. When sufficient nickel produces austenitic structures, steels become non-magnetic, ductile, and tough with reduced strength and elastic limit.
Commercial Applications
Carbon content intensifies nickel effects, with transformation temperatures varying according to carbon levels. Steels containing 2-5% nickel with approximately 0.1% carbon serve case hardening applications. Compositions with 0.25-0.40% carbon find use in crankshafts, axles, and connecting rods.
Low nickel steels achieve optimal properties through quenching and tempering treatments at 550-650°C. Reduced Ac₃ temperatures permit lower hardening temperatures and broader temperature ranges above Ac₃ without excessive grain growth, which nickel's slow diffusion rate helps prevent.
Special Nickel Alloy Applications
High nickel alloys serve specialized applications due to nickel's significant influence on thermal expansion coefficients. Alloys containing 36% nickel, 0.2% carbon, and 0.5% manganese exhibit practically zero thermal expansion between 0°C and 100°C. This composition, known as Invar, finds extensive use in precision instruments, clocks, measuring tapes, and thermal expansion regulators.
A carbon-free alloy containing 78.5% nickel and 21.5% iron demonstrates high magnetic permeability in weak magnetic fields, serving specialized electrical applications.
Chromium: Hardness and Wear Resistance
Carbide Formation and Heat Treatment
Chromium dissolves in both alpha and gamma iron phases, but carbon presence promotes complex carbide formation. These include cementite (FeCr)₃C with chromium contents up to 15%, and chromium carbides (CrFe)₃C₂, (CrFe)₇C₃, and (CrFe)₄C with varying chromium substitution levels. Stainless steels characteristically contain Cr₄C carbides.
Pearlitic chromium steels containing approximately 2% chromium demonstrate extreme sensitivity to cooling rates and heating temperatures before quenching. This sensitivity stems from chromium carbides' resistance to austenite dissolution, though dissolution increases with temperature.
Table 1. Critical hardening rates at different temperatures
| Temp. of Initial Heating, °C | Critical Hardening Rate (Mins to cool from 836° to 546°C) |
| 836 | 3,5 |
| 1010 | 6,5 |
| 1200 | 13 |
Microstructural Effects
Dissolved chromium raises critical transformation temperatures during both heating (Ac) and slow cooling (Ar). Faster cooling rates rapidly depress Ar temperatures, promoting steel hardening. Chromium produces characteristic modifications to isothermal transformation curve shapes.
Chromium reduces carbon percentage in pearlite structures, increasing the proportion of free cementite in high-carbon steels. Proper heat treatment produces spheroidized cementite forms more suitable for ball bearing applications. Chromium also refines pearlite structure for improved properties.
Corrosion Resistance and Applications
Chromium contents exceeding 1.1% in low-carbon steels form passive surface films that resist oxidizing attack. Higher chromium levels provide heat resistance in elevated temperature applications.
Chromium steels offer superior machinability compared to nickel steels of equivalent tensile strength. However, higher chromium contents create susceptibility to temper brittleness during slow cooling from tempering temperatures through 550-450°C ranges. These steels may also develop surface markings called "chrome lines."
Chrome steels serve applications requiring extreme hardness, including dies, ball bearings, safe plates, rolls, files, and cutting tools. High chromium content also appears in permanent magnet alloys.
Nickel-Chromium Combinations: Synergistic Effects
Combined Properties
While nickel steels excel in strength, ductility, and toughness, and chromium steels provide hardness and wear resistance, nickel-chromium combinations deliver all these properties with enhanced characteristics and reduced individual alloy disadvantages. The combination increases hardening depth, enabling air hardening in compositions with 4.5% nickel, 1.25% chromium, and 0.35% carbon.
Low nickel-chromium steels with minimal carbon content serve case hardening applications. Most structural applications utilize 0.25-0.35% carbon content with heat treatment to achieve desired properties. Significant nickel and chromium quantities provide corrosion and oxidation resistance at elevated temperatures.
Embrittlement Phenomena
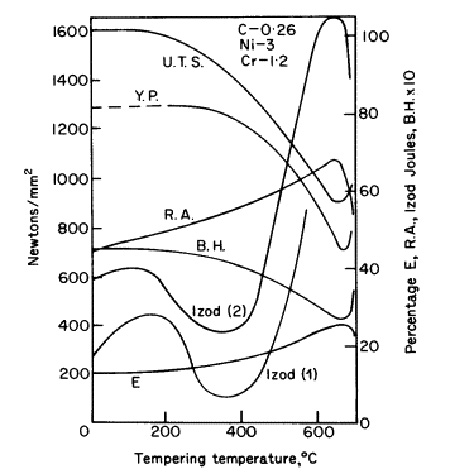
Figure 2: Effect of tempering on mechanical properties of nickel-chromium steel
Tempering effects in nickel-chromium steels reveal critical embrittlement zones. Impact testing shows dangerous minimums in the 250-450°C range, known as 350°C embrittlement. Phosphorus and nitrogen significantly influence this phenomenon, while other impurities (As, Sb, Sn) and higher manganese levels may contribute.
Temper brittleness describes notch impact intergranular brittleness induced by slow cooling after tempering above 600°C or prolonged exposure between 400-550°C. This phenomenon results from grain boundary enrichment with alloying elements (Mn, Cr, Mo) during austenitizing, leading to enhanced segregation of embrittling elements (P, Sn, Sb, As) through chemical interaction during slow cooling from 600°C.
Table 2. Cooling rate effects on steel properties
| Steel | Cooling Rate |
TS MPa |
Elongation | RA | Izod ft lbf |
Izod J |
| Ni-Cr | Oil | 896 | 18 | 60 | 64 | 87 |
| Ni-Cr | Furnance | 880 | 18 | 60 | 19 | 25 |
| Ni-Cr-Mo | Furnance | 896 | 18 | 61 | 59 | 80 |
Recovery from embrittlement occurs through reheating above 600°C followed by rapid cooling, redistributing and retaining embrittling segregation in solution. Molybdenum additions of 0.25% significantly reduce brittleness susceptibility.
Molybdenum: Hardenability and Temper Resistance
Molybdenum dissolves in both alpha and gamma iron phases, forming complex carbides including (FeMo)₆C, Fe₂₁Mo₂C₆, and Mo₂C in carbon's presence. Molybdenum effects on transformation kinetics resemble chromium but prove more effective up to 0.5% concentrations in retarding pearlite formation and promoting bainite development.
Plain carbon steels benefit from 0.5% molybdenum additions for increased strength at 400°C boiler temperatures. However, molybdenum primarily serves in combination with other alloying elements.
Ni-Cr-Mo steels find widespread use in ordnance, turbine rotors, and large structural components since molybdenum minimizes temper brittleness and reduces mass effects. Molybdenum also appears in high-speed steels, magnet alloys, and heat-resistant and corrosion-resistant compositions.
Additional Alloying Elements
Vanadium Applications
Vanadium functions as an oxide scavenger, forms V₄C carbides, and beneficially affects heat-treated steel properties, especially combined with other elements. It retards tempering in the 500-600°C range and can induce secondary hardening. Chromium-vanadium steels (0.15% V) serve locomotive forgings, automobile axles, coil springs, torsion bars, and creep-resistant applications.
Tungsten Characteristics
Tungsten dissolves in both gamma and alpha iron, forming WC and W₂C carbides, or Fe₃W₃C and Fe₄W₂C in iron's presence. The Fe₃W₂ compound provides age-hardening capabilities. Tungsten raises critical transformation temperatures and dissolves carbides slowly over temperature ranges.
Complete tungsten dissolution renders transformation sluggish, particularly tempering resistance utilized in hot-working tool steels and high-speed applications. Tungsten refines grain size and reduces decarburization tendencies during processing. Applications include magnets, corrosion-resistant, and heat-resistant steels.
Silicon Steel Applications
Silicon dissolves in ferrite as an effective hardening agent, raising Ac transformation temperatures and Ar temperatures during slow cooling while reducing gamma-alpha volume changes.
Three primary silicon steel types serve commercial applications:
- Silico-manganese springs: 0.5% C, 1.5% Si, 0.8% Mn
- Electrical silicon steel: 0.07% C, 4.3% Si, 0.09% Mn for transformer cores and electrical machinery requiring high magnetic permeability and electrical resistance
- Silichrome valve steel: 0.4% C, 3.5% Si, 8% Mn for automobile valves
Silicon contributes oxidation resistance in heat-resistant steels and serves general deoxidizing purposes.
Specialty Elements
Copper dissolves limitedly in ferrite with maximum 3.5% solubility at normalizing temperatures and 0.35% at room temperature. It lowers critical temperatures insufficiently for air-cooling martensite formation but improves atmospheric corrosion resistance and enables temper hardening.
Cobalt demonstrates high solubility in alpha and gamma iron with weak carbide-forming tendencies. It decreases hardenability but maintains hardness during tempering. Applications include Stellite-type alloys, gas turbine steels, magnets, and hard metal bonding.
Boron additions of 0.003-0.005% to fully killed, fine-grain steels increase hardenability significantly. This improves yield ratio and impact properties when combined with full hardening before tempering. Molybdenum-boron combinations create useful high-tensile bainitic steels. Boron also appears in hard-facing alloys and nuclear control rods.
Conclusion
Understanding alloying element characteristics enables steel engineers to design compositions meeting specific application requirements. The synergistic effects between elements often prove more valuable than individual contributions, particularly in complex alloy systems. Modern steel development continues optimizing element combinations to achieve superior performance while managing costs and material availability. Future developments will likely focus on micro-alloying techniques and advanced heat treatment processes to maximize beneficial effects while minimizing detrimental phenomena such as embrittlement and grain growth.
¡Encuentre al Instante la Composición Precisa de los Materiales!
Total Materia Horizon contiene la composición química de cientos de miles de materiales y sustancias, así como sus propiedades mecánicas y físicas, y mucho más.
Obtenga una cuenta de prueba GRATUITA de Total Materia Horizon y únase a nuestra comunidad que traspasa los 500.000 usuarios provenientes de más de 120 países.