Copper-Zinc Alloys: The Brasses
Abstract
This comprehensive analysis explores the composition, properties, and industrial applications of copper-zinc alloys, commonly known as brasses. The article examines how varying zinc content and processing methods influence the mechanical properties and microstructure of different brass types. Focusing on industrially significant compositions, from red brass to brazing solder, the study details the relationship between constituent phases (α, β, γ) and material characteristics. Special attention is given to manufacturing processes, including casting, rolling, and heat treatment, highlighting their effects on brass performance in engineering applications.
Introduction to Copper-Zinc Alloys
Copper alloys represent a versatile family of materials whose properties can be extensively modified through composition variation and treatment methods. In engineering applications, these alloys rank second only to steel in importance, primarily due to their adaptability and performance characteristics.
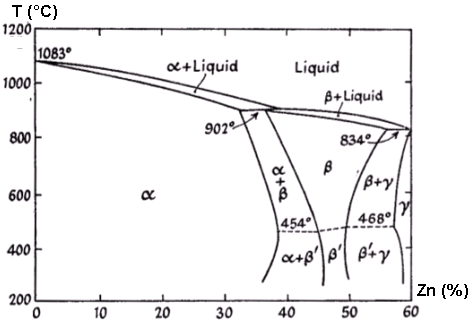
Figure 1: Constitutional diagram showing the relationship between copper-zinc composition and phase formation
Fundamental Properties and Phase Constituents
The addition of zinc to copper creates various solid solutions, designated as α, β, γ, in order to decrease copper content. The constitutional diagram demonstrates these phase relationships, with specific attention to industrially relevant compositions.
Table 1. Phase constituent table showing percentage composition and constituent formations
| Percentage composition | Constituent just below the freezing point | Constituent after slow cooling to 400°C | |
| Copper | Zinc | ||
| 100 to 67.5 | 0 to 32.5 | α | α |
| 67.5 to 63 | 32.5 to 37 | α + β | α |
| 63 to 61 | 37 to 39 | β | α |
| 61 to 55.5 | 39 to 45.5 | β | α + β` |
| 55.5 to 50 | 45.5 to 50 | β | β` |
| 50 to 43.5 | 50 to 56.5 | β | β` + γ |
| 43.5 to 41 | 56.5 to 59 | β + γ | β` + γ |
Compositional changes in the α and β' phases below 400°C occur only during extended annealing periods.
Mechanical Properties
The relationship between microstructure and mechanical properties follows distinct patterns that are crucial for industrial applications.
a. Tensile Strength:
- Increases proportionally with zinc content
- Shows sharp elevation upon β phase formation
- Peaks when α and β phases are present in equal proportions
- Decreases rapidly with γ phase appearance
b. Elongation Characteristics:
- Reaches maximum before α solution limit
- Decreases significantly with increasing β content
- Becomes minimal when γ phase is present
c. Impact Resistance:
The α phase demonstrates superior shock resistance, which progressively diminishes with β phase presence. The introduction of γ phase results in extreme brittleness.
d. Hardness Properties:
Material hardness significantly increases with β phase formation and further intensifies with γ phase presence.
Processing Characteristics
- α phase alloys: Ideal for cold working, suitable for both hot and cold rolling
- α+β alloys: Limited cold rolling capability, primarily suitable for hot rolling
- β phase alloys: Compatible with forging, rolling, and hot extrusion
- γ-containing alloys: Unsuitable for any mechanical processing
Industrial Brass Classifications and Properties
The brass of industrial significance is categorized according to their copper and zinc content, following the standardized UNS (Unified Numbering System) designation. The following compositions represent the most commercially significant brass alloys:
C 23000 (Red Brass - 85% Cu, 15% Zn)
This alloy is primarily utilized in ornamental pieces and affordable jewelry intended for gilding. In its as-cast condition, it exhibits a characteristic dendritic structure. When subjected to slow cooling or annealing, the material develops uniform polyhedral grains through diffusion. The process of diffusion is further enhanced through mechanical deformation of the grains by hot- or cold-work followed by annealing. These changes during rolling and annealing mirror those observed in 70:30 brass.
C 26000 (Cartridge Brass - 70% Cu, 30% Zn)
Widely employed in the production of tubes, sheets, and wires, this alloy displays a dendritic structure of the α solid solution when rapidly cooled. The β constituent emerges only when zinc content exceeds 32%, except when elements like aluminum or tin are present. After annealing, the alloy transforms into a homogeneous solid solution, making it particularly suitable for cold-working operations.
Manufacturing quality is paramount for this alloy. It requires the purest copper and zinc available, with minimal use of scrap material. The primary concerns are inclusions of dross (oxides or silicates) or charcoal, which can lead to failure during manufacturing or use. These inclusions typically become trapped during solidification, either through splashing or rapid solidification in small cross-section molds.
The casting process requires careful consideration of mold dimensions and pouring speeds to ensure proper solidification from bottom upwards. Cold-working breaks down the crystal grains through plastic deformation, while subsequent annealing promotes recrystallization and grain growth.
C 28000 (Muntz Metal - 60% Cu, 40% Zn)
This alloy begins solidification at approximately 905°C, forming β solution dendrites. During slow cooling, the homogeneous β constituent undergoes transformation at 770°C, where the α constituent separates and increases in proportion as temperature decreases. The final structure consists of an α and β mixture, whose proportions can be controlled through cooling rates.
When hot-rolled above 700°C, the alloy displays a uniform structure in both longitudinal and transverse directions. The finishing temperature directly influences the final grain size, with lower temperatures resulting in finer grains. Rolling below 600°C may impede recrystallization, leading to cold-work effects in the metal.
Brazing Solder (50% Cu, 50% Zn)
This composition represents the maximum practical zinc content for brass alloys. When cooled slowly through solidification, it forms a homogeneous β solution. However, zinc content exceeding 50% triggers the formation of the γ constituent, which increases with decreasing temperature. The presence of γ phase results in extreme hardness and brittleness, making precise composition control essential during manufacturing.
This alloy requires careful temperature control during processing, as the presence of impurities can affect the maximum zinc content that can be retained in the β solution at room temperature. The formation of γ phase must be avoided to maintain workable properties.
Jetzt Zugang zu präzisen Eigenschaften von Kupferlegierungen!
Total Materia Horizon enthält Eigenschaftsinformationen für mehr als 30.000 Kupferlegierungen: Zusammensetzung, mechanische, physikalische und elektrische Eigenschaften, nichtlineare Eigenschaften und vieles mehr.
Holen Sie sich ein KOSTENLOSES Testkonto bei Total Materia Horizon und schließen Sie sich einer Gemeinschaft von über 500.000 Benutzern aus mehr als 120 Ländern an.