Clean Steel: Part One
Abstract
Steel cleanliness represents a critical factor in determining steel quality, with increasing industry demand for cleaner steels annually. Clean steel is characterized by exceptionally low content of impurity elements including phosphorus, sulfur, total oxygen, nitrogen, hydrogen, and sometimes carbon, along with minimal non-metallic inclusions. Achieving satisfactory steel cleanliness requires comprehensive control throughout steelmaking processes, including deoxidant and alloy additions, secondary metallurgy treatments, shrouding systems, and casting practices. The specific cleanliness requirements vary significantly among steel grades, with applications ranging from automotive sheets requiring carbon content below 30 ppm to tire cord steel demanding inclusion sizes smaller than 10 µm. Steel producers must implement precise metallurgical control strategies to meet these stringent cleanliness standards and deliver superior mechanical properties.
Introduction to Steel Cleanliness Standards
Steel cleanliness has emerged as a fundamental factor determining steel quality, with the demand for cleaner steels increasing consistently year after year. Clean steel is generally defined as steel containing extremely low levels of impurity elements, including phosphorus, sulfur, total oxygen, nitrogen, hydrogen, and occasionally carbon, alongside minimal non-metallic inclusions. The improvement of steel cleanliness has consequently become an increasingly important subject in ferrous metallurgical technology development and represents a critical task for iron and steel producers worldwide.
The growing demand for superior mechanical properties in steel applications has driven steel producers to continuously improve the cleanliness of their final products. Achieving satisfactory steel cleanliness requires controlling and improving a comprehensive range of operating practices throughout steelmaking processes, including deoxidant and alloy additions, secondary metallurgy treatments, shrouding systems, and casting practices.
Defining Clean Steel Categories
Due to the somewhat vague nature of the term "clean steel," some metallurgical experts suggest more precise terminology. They propose referring to steels with low solute levels as "high purity steels," steels with minimal impurities originating from remelting scrap as "low residual steels," and steels with low frequency of product defects related to oxide presence as "clean steels."
Impact of Impurity Elements on Steel Properties
The individual or combined effects of carbon [C], phosphorus [P], sulfur [S], nitrogen [N], hydrogen [H], and total oxygen (T.O.) in steel can significantly influence steel properties. These properties include tensile strength, formability, toughness, weldability, cracking resistance, corrosion resistance, and fatigue resistance. Additionally, clean steel production requires careful control of non-metallic oxide inclusions, including their size distribution, morphology, and composition.
The control requirements for these elements differ according to specific performance demands and vary among different steel grades. Some elements that prove harmful to certain steel grades may be less detrimental or even beneficial to other grades.
Table 1. Influence of typical impurities on mechanical properties
| Element | Form | Mechanical Properties Affected |
| S, O | Sulfide and oxide inclusions |
|
| C, N | Solid solution |
|
| Settled dislocation |
|
|
| Pearlite and cementite |
|
|
| Carbide and nitride precipitates |
|
|
| P | Solid solution |
|
For instance, Interstitial-Free (IF) steels require carbon, nitrogen, total oxygen, and inclusion content to be as low as possible to achieve good flexibility, high "r" value, and perfect surface quality. Conversely, high-quality pipeline steel demands ultra-low sulfur, low phosphorus, low nitrogen, low total oxygen content, and a specific calcium-to-sulfur ratio.
Steel Grade-Specific Cleanliness Requirements
Steel cleanliness depends significantly on the amount, morphology, and size distribution of non-metallic inclusions. These inclusions generate numerous defects, and many applications restrict the maximum allowable inclusion size, making the size distribution of inclusions in steel products critically important. For applications requiring stringent mechanical properties, internal steel cleanliness becomes paramount.
Table 2. Cleanliness requirements for various steel grades
| Steel product | Maximum allowed impurity fraction | Maximum allowed inclusion size |
| IF steels | [C]≤30 ppm, [N]≤40 ppm, T.O.≤40 ppm [C]≤10 ppm, [N]≤50 ppm |
|
| Automotive and deep-drawing Sheets | [C]≤30 ppm, [N]≤30 ppm | 100 µm |
| Drawn and Ironed cans | [C]≤30 ppm, [N]≤40 ppm, T.O.≤20 ppm | 20 µm |
| Alloy steel for Pressure vessels | [P]≤70 ppm | |
| Alloy steel bars | [H]≤2 ppm, [N]≤20 ppm, T.O.≤10 ppm | |
| HIC resistant steel sour gas tubes | [P]≤50 ppm, [S] ≤10 ppm | |
| Line pipes | [S]≤30 ppm, [N]≤50 ppm, T.O.≤30 ppm | 100 µm |
| Sheets for continuous annealing | [N]≤20 ppm | |
| Plates for welding | [H]≤1.5 ppm | |
| Bearings | T.O.≤10 ppm | 15 µm |
| Tire cord | [H]≤2 ppm, [N]≤40 ppm, T.O.≤15 ppm | 10 µm |
| Non-grain-orientated Magnetic Sheets | [N]≤30 ppm | |
| Heavy plate steels | [H]≤2 ppm, [N]=30-40 ppm, T.O.≤20 ppm | Single inclusion 13 µm Cluster 200 µm |
| Wires | [N]≤60 ppm, T.O.≤30 ppm | 20 µm |
Automotive and Deep-Drawing Applications
For sheets used in automotive body applications, carbon [C], nitrogen [N], and total oxygen (T.O.) must each be maintained at very low levels. Automotive and deep-drawing sheets require carbon and nitrogen content below 30 ppm, with maximum inclusion sizes limited to 100 µm.
Specialized Industrial Applications
For sheets destined for tin plate applications, total oxygen must not only remain below 20 ppm, but the size of non-metallic inclusions must be less than 20 µm. Steel cord used in tire manufacturing demands inclusion sizes smaller than 10 µm, while TV shadow mask applications require even smaller inclusions (5 µm).
Ball bearing steel applications require total oxygen content below 10 ppm and inclusion sizes smaller than 15 µm to improve fatigue-resistance properties. Petroleum pipeline applications and Hydrogen Induced Cracking (HIC) resistant steels for sour natural gas transport require extremely low sulfur [S] content, typically less than 10 ppm.
Steelmaking Process Control for Clean Steel Production
Steel cleanliness is controlled through a comprehensive range of operating practices throughout steelmaking processes. These practices encompass the timing and location of deoxidant and alloy additions, the extent and sequence of secondary metallurgy treatments, stirring and transfer operations, shrouding systems, tundish geometry and practices, the absorption capacity of various metallurgical fluxes, and casting practices.
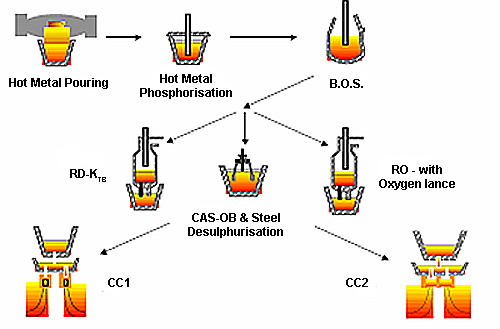
Figure 1: is positioned here to outline the process route for clean steel production
The steelmaking process route for clean steel production involves multiple stages of refinement and control, each contributing to the final cleanliness level achieved in the steel product. This integrated approach ensures that impurity levels are minimized and inclusion characteristics are optimized for specific applications.
Conclusion
The production of clean steel represents a complex metallurgical challenge requiring precise control throughout the entire steelmaking process. As industry demands for superior steel properties continue to increase, steel producers must implement comprehensive strategies to achieve the stringent cleanliness standards required for modern applications. Understanding the specific requirements for different steel grades and implementing appropriate process controls remains essential for successful clean steel production.
立即查看精确的化学成分!
Total Materia Horizon 包含数十万种材料和物质的化学成分,以及机械和物理性能等。

申请 Total Materia Horizon免费试用帐户,加入来自全球 120 多个国家超过 500,000 名用户的大家庭。