Temper Embrittlement
Abstract
Temper embrittlement is a crucial phenomenon in steel manufacturing that affects material toughness without significantly impacting other mechanical properties at room temperature. This article examines two distinct temperature intervals of embrittlement: irreversible (250-400°C) and reversible (450-650°C). The process, influenced by various alloying elements and impurities, particularly affects commercial steels containing elements like chromium, nickel, and manganese. Understanding and preventing temper embrittlement is essential for maintaining steel quality and performance in industrial applications.
Types and Temperature Ranges of Temper Embrittlement
Temper embrittlement is inherent in many steels and is characterized by reduced impact toughness. At room temperature, the state of temper embrittlement has virtually no effect on other mechanical properties. Many alloy steels exhibit two distinct temperature intervals of temper embrittlement: irreversible temper brittleness within 250-400°C and reversible temper brittleness within 450-650°C.
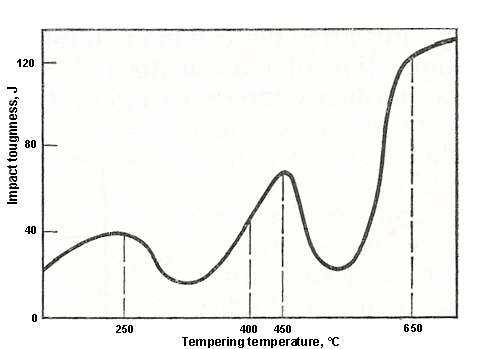
Figure 1: Impact toughness vs. tempering temperature
The impact toughness of quenched steel after tempering at 250-400°C is lower than that achieved by tempering at temperatures below 250°C. When brittle steel tempered at 250-400°C is heated above 400°C and transformed into a tough state, subsequent tempering at 250-400°C cannot restore its brittle state. The cooling rate from the tempering temperature within 250-400°C does not affect impact toughness.
Mechanisms of Embrittlement
Irreversible Embrittlement
Steel in the state of irreversible temper embrittlement displays a bright intercrystalline fracture at the boundaries of former austenitic grains. This type of brittleness is present to some degree in all steels, including carbon grades. For this reason, medium-temperature tempering is generally avoided in practice, despite its ability to ensure a high yield limit.
The formation of carbides during martensite decomposition, particularly their precipitation as films at grain boundaries, is believed to cause irreversible temper embrittlement. These films dissolve at higher tempering temperatures and cannot reform during subsequent heating at 250-400°C. In low-alloy steels, silicon can prevent irreversible temper embrittlement by slowing martensite decomposition.
High-Temperature Embrittlement
High-temperature tempering embrittlement manifests in two ways:
- Through heating at 450-600°C (regardless of subsequent cooling rate)
- Through tempering above 600°C followed by slow cooling within 600-450°C
Role of Alloying Elements and Impurities
Carbon steels containing less than 0.5% Mn are resistant to reversible temper embrittlement. This phenomenon appears exclusively in alloy steels. Common alloying elements like chromium, nickel, and manganese promote temper embrittlement, with their combined effect being more pronounced than their individual impacts. Cr-Ni and Cr-Mn steels show the most significant embrittling effect. Small molybdenum additions (0.2-0.3%) can reduce temper embrittlement, while larger amounts intensify it.
High-purity alloy steels show no susceptibility to temper embrittlement. The phenomenon occurs due to impurities present in commercial steels, primarily phosphorus, tin, antimony, and arsenic.
Prevention and Control Measures
To prevent temper embrittlement, the following measures are recommended:
- Minimizing harmful impurity content in steel
- Implementing accelerated cooling from high-temperature tempering (above 600°C)
- Adding small amounts of molybdenum (0.2-0.3%)
- Applying high-temperature thermo-mechanical treatment
Scientific Research and Findings
Historical Theories
For many years, scientists supported the "solution precipitation" hypothesis, which attributed the loss in impact toughness to the precipitation of phases such as phosphides at grain boundaries. These phases were thought to enter the α-solution when heated to approximately 650°C and precipitate during slow cooling, causing embrittlement. However, electron-microscopic analysis revealed no special precipitates at grain boundaries in embrittled steel, disproving this hypothesis.
Modern Understanding
Recent research has established that temper embrittlement occurs due to increased impurity concentration in boundary layers of the solid solution. This finding is supported by:
- Enhanced etchability of grain boundaries in embrittled steel using picric acid
- Advanced analysis using Auger spectroscopy, which can detect element concentrations in monatomic surface layers
- Direct correlation between embrittlement development and impurity concentration near prior austenite boundaries
Impurity Segregation Mechanisms
The concentration of harmful impurities at fracture surfaces can exceed their average steel concentration by factors of tens or hundreds. While commercial purity steels typically contain only thousandths or hundredths of a percent of impurities, these concentrations can reach several percent at fracture surfaces.
Temperature plays a crucial role in the segregation process:
- Higher temperatures accelerate grain boundary segregation
- Thermal motion simultaneously reduces equilibrium segregation
- Above 600-650°C, impurity segregation either disappears completely (Sb) or becomes negligible (P)
- Rapid water cooling prevents segregate restoration
Interaction Between Alloying Elements and Impurities
The relationship between alloying elements and impurities is complex:
- Iron-carbon alloys alone show minimal impurity segregation
- Alloying elements (Ni, Cr, Mn) significantly increase impurity segregation
- Alloying elements themselves segregate at grain boundaries when harmful impurities are present
- Mutual interaction between impurities and alloying elements enhances segregation
Specific Interactions
Strong atomic attractions between impurities and alloying elements lead to enhanced mutual segregation in pairs such as:
- Phosphorus and Nickel
- Phosphorus and Chromium
- Antimony and Nickel
- Antimony and Manganese
Multiple alloying elements can create synergistic effects. For example, the combination of nickel and chromium causes greater antimony segregation than the sum of their individual effects.
Prevention and Control Strategies
- Impurity Control
- Strict control of harmful impurity content during steel production
- Regular monitoring of phosphorus, tin, antimony, and arsenic levels
- Heat Treatment Optimization
- Accelerated cooling from high-temperature tempering (above 600°C)
- Careful control of cooling rates through critical temperature ranges
- Alloying Strategies
- Strategic use of molybdenum additions (0.2-0.3%)
- Careful balance of necessary alloying elements
- Consideration of element interactions
- Processing Techniques
- Implementation of high-temperature thermo-mechanical treatment
- Optimization of processing parameters based on steel composition
Conclusion
Understanding temper embrittlement is crucial for steel manufacturing and application. Through proper control of composition, processing parameters, and heat treatment procedures, the negative effects of temper embrittlement can be effectively managed.
材料の正確な特性を即座に検索!
Total Materia Horizon には、数十万種類の材料の機械的・物理的特性が、異なる温度・条件・熱処理などに応じて収録されています。
Total Materia Horizonの無料テストアカウントを開設して、120カ国以上、50万人を超えるユーザーのコミュニティに参加しましょう!