Slag Metal Reactions in Welding: Part Two
Abstract
As the steel technologies have increased the possibilities to make new and improved materials with excellent mechanical and corrosion properties the need for matching advancements in weld properties has become increasingly apparent.
Slag-melt reactions particularly relating to Cr and Si hold some key information for driving these improvements.
During the past three decades there has been tremendous progress in steel technology leading to many alloys possessing excellent mechanical and corrosion resistant properties. In many cases it is necessary to join these materials by welding techniques which are capable of achieving similar properties.
The aim of an investigation presented in the paper of Mitra U. et al. (1984) was to determine the extent of the interaction between slags and weld metals containing chromium, molybdenum, and nickel in addition to carbon, silicon, manganese, sulfur and phosphorus. This study utilizes the concept of an equilibrium or neutral point as developed by Chai and provides an estimate of the effective reactions temperature between the slag and the metal.
The transfer of Cr, Si, Mn, P, S, Ni, and Mo between slag and the weld pool has been studied for submerged arc welds made with different fluxes. The results show a strong interaction between Cr and Si transfer but no interaction with Mn.
The effect of slag-metal reactions on the transfer of various elements present in low alloy and stainless steels has resulted in the following conclusions:
- The transfer of chromium is strongly dependent on the type of flux used. Lime silicate fluxes produced weld metal with much higher chromium content than manganese silicate fluxes, although both fluxes contained the same amount of chromium (III) oxide.
- The transfer of silicon is greatly influenced by the basicity index of the flux used by the initial chromium content of the electrode, the greater will be the amount of silicon in the weld metal.
- The manganese content of the weld metal depends mainly on the amount of manganese oxide in the flux and the initial manganese content of the electrode. The amount of the other alloying elements present does not appear to have significant influence on the transfer of manganese.
- The desulfurizing capability of a flux should be judged by the flux type, the basicity index, and the initial amount of sulfur present in the flux. The MnO-Cr2O3-SiO2 flux (Basicity index=0.35) gave slightly better desulfurization than CaO-Cr2O3-SiO2 type fluxes (Basicity index = 0.8-1.0).
On the other hand in the paper of Lau T and al., a detailed study is presented of the interactions of Mn, Al and O at the different stages of the welding operation. These interactions have been studied by analyzing the total Mn, Al and O contents, as well as the composition of inclusions formed at the different stages. At the electrode tip and in the arc column, changes in oxygen, aluminum and manganese were dominated by flux decomposition, while at the weld metal stage, slag-metal reactions occurred.
These reactions were most extensive in MnO containing fluxes, and resulted in a significant loss of oxygen, as well as metallic species such as aluminum, by the separation of oxidized products into the slag phase. In the CaF2-Al2O3 containing flux studied, reactions involving CaF2, AI2O3 and dissolved Al and Si led to enhanced Al pickup at the electrode tip. The applicability of equilibrium thermodynamic arguments to welding was assessed by determining the effective temperatures for the pertinent equilibrium reactions. These temperatures were found to be in reasonable agreement with other values in the literature. However, it is the efficiency of inclusion separation to the slag that determines the effective temperature.
Five synthetic laboratory fluxes were used for this study. The composition of the fluxes, together with the wire and base plate, are given in Table 1.
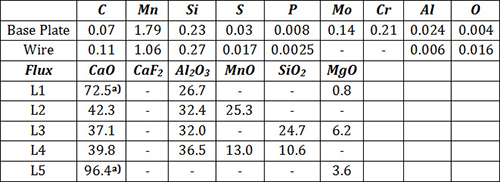
Table 1: Compositions of Base Plate, Wire and Flux used in the study
Only Ca was determined by x-ray fluorescence analysis. Flux L1 contained 37.5% CaO, 25, CaF2 and 37.5% Al2O3, while Flux L5 contained 25% CaO and 75% CaF2 prior to melting.
A description of the preparation of the fluxes, welding procedures and analysis of the electrode tips, weld metal droplets and weld metal was given in the previous study of Lau T et al. All welds were made either at 300 or 600 A with the same heat input. The total Al and Mn contents of the samples were analyzed by neutron activation analysis. A sample weighing about 0.5 g (0.018 oz) was irradiated for 30 s at an energy level of 2 kW (1011 neutrons).
In the analyzed study, the oxygen, aluminum and manganese contents, as well as the inclusion compositions at the electrode tip, metal droplet and weld metal stages, have been determined. With fluxes, wire and base plate of known compositions, and from the relative changes from one stage to another, it was possible to assess and evaluate the different types of reactions occurring during the weld process and to identify the major factors which control the final oxygen level in the weld metal. The following points summarize the observations:
1. The electrode tip and metal droplet stage are the major sites for oxygen absorption. Determination of aluminum and manganese level, as well as the composition of inclusions, showed that the major source of oxygen is decomposition of the flux.
2. It was found that a significant portion of the aluminum absorbed in the electrode tip and droplet stages was in the solid solution form, i.e., a nonoxidized form. This implied extensive decomposition of flux components within the arc column.
3. At the weld metal stage, metal-slag reactions may or may not occur. In this study, it was found that fluxes containing MnO showed significant metal-slag reactions, while MnO-free fluxes did not. Significant loss of oxygen, as well as metallic species such as aluminum, at the weld metal stage indicated separation of oxidation products as the most significant factor determining the final oxygen content.
5. A near equilibrium state may be attained at some stage during the thermal cycle of the weld pool; however, it is the efficiency of inclusion separation that determines the final oxygen level in the weld metal.
数千種類の溶接材料を即座に検索!
Total Materia Horizon には、溶接に適した数千種類の材料や電極のデータ(母材と溶接後の状態の両方)が収録されています。
Total Materia Horizonの無料テストアカウントを開設して、120カ国以上、50万人を超えるユーザーのコミュニティに参加しましょう!