Precipitation Hardening of Aluminum Alloys
Abstract
Precipitation hardening, or age hardening, provides one of the most widely used mechanisms for the strengthening of metal alloys. The strongest aluminum alloys (2xxx, 6xxx and 7xxx) are produced by age hardening.
In order for an alloy system to be able to be precipitation-strengthened, there must be a terminal solid solution that has a decreasing solid solubility as the temperature decreases. The precipitation-hardening process involves three basic steps: solution treatment, quenching and aging.
Introduction to Precipitation Hardening
Precipitation hardening, or age hardening, is a widely used mechanism for strengthening metal alloys. The strongest aluminum alloys (2xxx, 6xxx, and 7xxx) are produced through this process, which involves three basic steps: solution treatment, quenching, and aging.
Historical Background
The foundation for precipitation hardening was established by early work at the U.S. Bureau of Standards on Duralumin. Discovered accidentally by Wilm between 1903 and 1911, age hardening quickly became significant commercially under the trade name Duralumin.
Mechanism of Strengthening
The strength and hardness of some metal alloys, such as aluminum, copper-tin, certain steels, nickel-based super-alloys, and titanium alloys, can be enhanced by forming small, uniformly dispersed second-phase particles within the original phase matrix. These particles act as obstacles to dislocation movement, strengthening the alloys.
Requirements for Precipitation Hardening
An alloy system must have a terminal solid solution with decreasing solid solubility as temperature decreases. The Al-Cu (Duralumin) phase diagram demonstrates this, where a large decrease in solid solubility occurs from 550°C to 75°C.
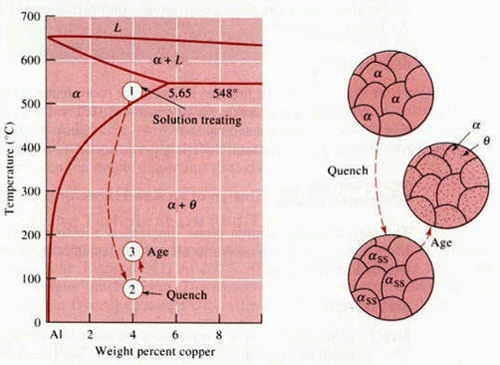
Figure 1: The aluminum-rich end of the Al-Cu phase diagram showing the three steps in the age-hardening heat treatment and the microstructures that are produced.
Process of Precipitation Hardening
The process involves three basic steps:
- Solution Treatment (Solutionizing)
- The alloy is heated above the solvus temperature to produce a homogeneous solid solution (α). This dissolves the θ precipitates and reduces segregation in the original alloy.
- Quenching
- The solid α is rapidly cooled to form a supersaturated solid solution of αSS, which contains excess copper and is not an equilibrium structure. This prevents θ precipitates from forming.
- Aging
- The supersaturated α, αSS, is heated below the solvus temperature to produce finely dispersed precipitates. These precipitates impede dislocation movement, thereby strengthening the alloy.
Historical Development
Paul D. Merica and coworkers' research on Duralumin revealed significant findings:
- Solubility of CuAl2 in aluminum increases with temperature
- The hardening is caused by precipitation of CuAl2 in fine molecular, colloidal, or crystalline form
- The particle size of CuAl2 affects the hardening
Steps of Precipitation Hardening
| Step | Description |
|---|---|
| Solution Treatment | Heating the alloy above the solvus temperature to form a homogeneous solid solution (α). |
| Quenching | Rapid cooling to form a supersaturated solid solution (αSS). |
| Aging | Heating the supersaturated α to form finely dispersed precipitates that impede dislocation movement. |
Age Hardening in Aluminum Alloys
The strongest aluminum alloys (2xxx, 6xxx, and 7xxx) are produced by age hardening. Fine dispersion of precipitates is formed through appropriate heat treatment.
Precipitation Sequences in Aluminum Alloys
The general model for decomposition and precipitation sequences in aluminum alloys is:
a0 (SSSS) → GP zones → θ'' → →θ' → θ or, more fully:
a0 (SSSS) → α1 + GP zones → α2 + θ'' → α3 + θ' → α4 + θ
Age Hardening Mechanisms
The three main mechanisms of strengthening through age hardening are:
- Coherency Strain Hardening
- Interaction between dislocations and strain fields around GP zones or coherent precipitates.
- Chemical Hardening
- Increased stress required for dislocations to cut through coherent precipitates.
- Dispersion Hardening
- Increased shear stress needed for dislocations to bypass incoherent precipitates or particles.
Detailed Understanding of Precipitation Reactions
Precipitation reactions in Al-Cu alloys are complex:
- The equilibrium phase CuAl2 is difficult to nucleate, leading to the formation of metastable precipitates.
- GP zones (GP1 and GP2) develop into θ' precipitates, eventually replaced by equilibrium phase CuAl2, which contributes little to hardness.
Process Models for Precipitation Hardening
- 6000 Series Aluminum Alloys: Models describe the effect of quench-induced precipitation on hardening during isothermal low-temperature aging.
- 7000 Series Aluminum Alloys: Fracture toughness is related to microstructure elements resulting from thermo-mechanical treatments.
Challenges and Limitations
A comprehensive description of the simultaneous operation of multiple precipitation phenomena is still lacking. Understanding the competitive precipitation of several phases on various nucleation sites and predicting fracture toughness in complex situations remains a challenge.
Summary
Precipitation hardening is a crucial process for strengthening aluminum alloys, involving solution treatment, quenching, and aging. The mechanisms and effects of this process have been extensively studied and applied to develop high-strength aluminum alloys used in various industries. However, challenges remain in fully understanding and modeling the complex interactions in precipitation hardening.
数千種類の熱処理図を即座に検索!
Total Materia Horizon には、数十万種類の材料の熱処理データ(焼入れ性曲線、焼戻し硬度データ、TTT・CCT図など)が収録されています。

Total Materia Horizonの無料テストアカウントを開設して、120カ国以上、50万人を超えるユーザーのコミュニティに参加しましょう!