Platinum and Its Use
Abstract
Platinum, a rare and valuable precious metal, plays a crucial role in modern industry and technology. This comprehensive article explores platinum's physical properties, chemical characteristics, and diverse applications across multiple sectors. From its vital role in automotive catalytic converters to its use in jewelry and electronics, platinum's unique properties make it indispensable in contemporary manufacturing. The article details how platinum's resistance to corrosion, excellent catalytic properties, and durability contribute to its high value and widespread industrial use, while also examining its significance in emerging technologies and investment markets.
Physical Properties and Chemical Characteristics
Platinum (Pt, atomic number 78) is a dense, malleable, ductile, precious gray-white transition metal. Found in extremely low concentrations of 0.003 ppm in Earth's crust, it was first discovered in South America, with its name derived from the Spanish term "platina del Pinto" (little silver of the Pinto River). Discovered by Ulloa in 1753 and Wood in 1741, this metal was initially used by pre-Columbian Indians.
As a Group 10 element, platinum demonstrates remarkable resistance to corrosion and occurs naturally in nickel and copper ores. It's typically found alongside other precious metals including iridium, osmium, palladium, ruthenium, and rhodium in alluvial deposits of the Ural Mountains, Colombia, and various Western American states.
Chemical Properties and Isotopes
Pure platinum exhibits a beautiful silvery-white appearance with exceptional malleability and ductility. Its coefficient of expansion closely matches that of soda-lime-silica glass, making it ideal for sealed electrodes in glass systems. While the metal resists oxidation at any temperature, it can be corroded by halogens, cyanides, sulfur, and caustic alkalis. Though insoluble in hydrochloric and nitric acid individually, it dissolves in aqua regia to form chloroplatinic acid (H2PtCl6).
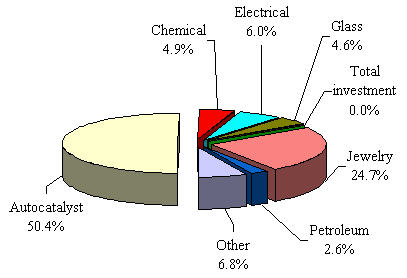
Figure 1. Platinum demand by application in 2006
Industrial Applications
Automotive and Environmental
The most significant application of platinum is in catalytic converters, where it transforms harmful emissions into harmless substances. Approximately half of newly mined platinum serves this purpose, with demand increasing due to stricter global emission standards.
Jewelry and Precious Metals
Platinum jewelry accounts for approximately 25% of total platinum demand. Its durability, purity (95% in Europe and USA), and resistance to tarnish make it ideal for fine jewelry and settings for precious stones.
Technology and Electronics
In the technology sector, platinum plays a crucial role in:
- Hard disk drive coatings
- Fiber optic cables
- Thermocouples
- Multi-layer ceramic capacitors
Additional Applications
- Petroleum refining
- Medical devices and anti-cancer drugs
- Fuel cells (PEM)
- Investment vehicles
- Glass manufacturing
- Chemical processing
Market Value and Investment
Platinum maintains significant value as both an industrial metal and an investment vehicle. With the ISO currency code XPT, its price fluctuates according to market forces. Its value is supported by its relative scarcity, historical performance, and fundamental industrial importance.
材料の正確な特性を即座に検索!
Total Materia Horizon には、数十万種類の材料の物理特性、熱特性、電気特性が、異なる温度などに応じて収録されています。
Total Materia Horizonの無料テストアカウントを開設して、120カ国以上、50万人を超えるユーザーのコミュニティに参加しましょう!