The Queneau-Schuhmann-Lurgi (QSL) Process
Abstract
The QSL process represents one of the most promising lead processing technologies, effectively addressing the drawbacks of conventional sinter plant-blast furnace methods. This highly efficient process produces lead from lead concentrates and secondary raw materials in a single reactor, reducing environmental impact by half compared to conventional methods. The technology utilizes oxygen instead of air and exploits sulfide sulfur as the main energy source, significantly reducing fossil fuel consumption and CO2 emissions. With energy requirements dropping from 15.2 to 4.5 GJ per ton of lead produced, the QSL process demonstrates superior efficiency while maintaining high production standards of 155,000 tons of lead annually.
Introduction to Lead Production
Lead stands among the few metals known and utilized by humanity prior to 5000 B.C. The only commercially important lead mineral is galena (PbS), a dark gray sulfide characterized by bright metallic luster and cubic habit. Lead may have been the first metal produced by smelting, as the required operating temperature of less than 900°C (compared to copper's requirement of over 1,100°C) can be easily achieved in a simple open wood fire. The metal's distinctive properties include softness, heaviness, deformability without rupture, corrosion resistance, and a low melting point of 327°C.
Raw Materials and Production Methods
Lead production utilizes various primary and secondary raw materials. Sulfidic lead ore concentrates serve as the main primary raw material, while lead-acid batteries constitute the most important secondary raw material. Additionally, recycled oxidized and metallic products from other metallurgical operations undergo processing.
Distinguishing between primary and secondary lead production processes often proves difficult, as many plants utilize both primary and secondary raw materials. On an industrial scale, only pyrometallurgical processes operate for lead production, while hydrometallurgical production of lead has not achieved commercial scale implementation.
Evolution of Lead Processing Technologies
During recent decades, several innovative lead production processes have emerged to overcome the drawbacks of the conventional sinter plant-blast furnace route. The most promising developments include flash smelting and bath smelting processes. Among these, the QSL (Queneau-Schuhmann-Lurgi) technology, Kivcet technology, and TBRC process have proven their industrial-scale suitability.
Currently, three industrial plants worldwide operate the QSL process: Berzelius Metall GmbH in Stolberg, Germany; Korea Zinc in Onsan, South Korea; and CNIEC in China. The Kivcet process operates in Italy (Nuova Samim, Sardinia), Kazakhstan (UK Lead and Zinc Combine), and Canada (Trail, British Columbia).
QSL Process Technology Overview
The QSL process, named after inventors Queneau, Schumann, and Lurgi, enables highly efficient lead production from lead concentrates and secondary raw materials in one reactor. Unlike conventional equipment, this innovative technology utilizes oxygen instead of air and employs sulfide sulfur existing in concentrates as the main energy source. This approach allows major elimination of fossil fuels and significantly reduces environmental impact, with CO2 emissions representing only half the volume of conventional processes.
The process generates steam from waste heat of process waste gas containing sulfur dioxide, which feeds into a turbine powering an electricity-producing generator. Subsequently, the process gas undergoes cleaning by state-of-the-art filter units before the contained sulfur dioxide converts to exceptionally pure sulfuric acid in the sulfuric acid unit.
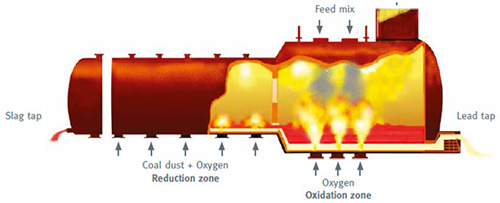
Figure 1: QSL furnace
Treatment Process
The treatment process involves homogenizing sulfide-containing lead ores and secondary raw materials with coal and water in continuous mixers. These materials then undergo smelting in a 33-meter long horizontally positioned QSL reactor divided into an oxidation zone (3.5 meters in diameter) and a reduction zone (3 meters in diameter).

Figure 2: QSL reactor
Smelting Operations
Conveyor belts feed the premixed charge through feed ports to the reactor. Pure oxygen injection occurs through tuyères at the reactor bottom at temperatures of 1,200°C. The resulting oxidation zone produces lead bullion containing impurities of copper, silver, other precious metals, antimony, and bismuth. Due to the slightly inclined construction, lead bullion at temperatures exceeding 1,000°C flows to the front end of the oxidation zone. The off-gas undergoes cooling to 400°C, multi-step cleaning, and conversion of contained SO2 to exceptionally high-purity sulfur dioxide.
Primary slag containing residual lead in lead oxide form also forms in the oxidation zone and flows counter-currently into the reduction zone. Coal dust charged in this zone reduces lead oxide in the slag to metallic lead, which then flows back to the oxidation zone.
Tapping and Material Recovery
Siliceous slag extraction occurs from the rear end of the reduction zone, followed by quenching using powerful water jets to create sand with 1mm grain size. This glassy granulate carries the trade name BERZELIT. Metallic crude lead undergoes continuous siphoning from the front end of the oxidation zone into cooling kettles where decopperization takes place. Further treatment continues in the refining process.
Refining Process
Lead refining occurs in 24 kettles, where individual impurities of the lead bullion undergo selective removal in up to nine steps—a considerably more complicated procedure than in secondary smelters. The produced lead achieves exceptionally high purity grades and serves as starting material for creating precisely defined lead alloys through specific additions of copper, calcium, tin, silver, or tellurium.
Process Efficiency and Environmental Benefits
The QSL technology enables lead extraction from lead ores and secondary raw materials in a single encapsulated unit, demonstrating noticeably lower specific energy consumption compared to conventional technologies. Energy requirements decreased dramatically from 15.2 to 4.5 GJ per ton of lead produced by exploiting sulfide energy contained in ores as the main energy source. The wide range of chargeable materials underscores process efficiency, with annual production of 155,000 tons of lead and its alloys, plus 120,000 tons of sulfuric acid, setting distinctive international standards.
Accédez en quelques instants à des milliers de courbes de traitement thermique !
Total Materia Horizon contient des informations de traitement thermique pour des centaines de milliers de matériaux, diagrammes de trempabilité, courbe de revenu, TTT et CRT, et bien plus.

Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.