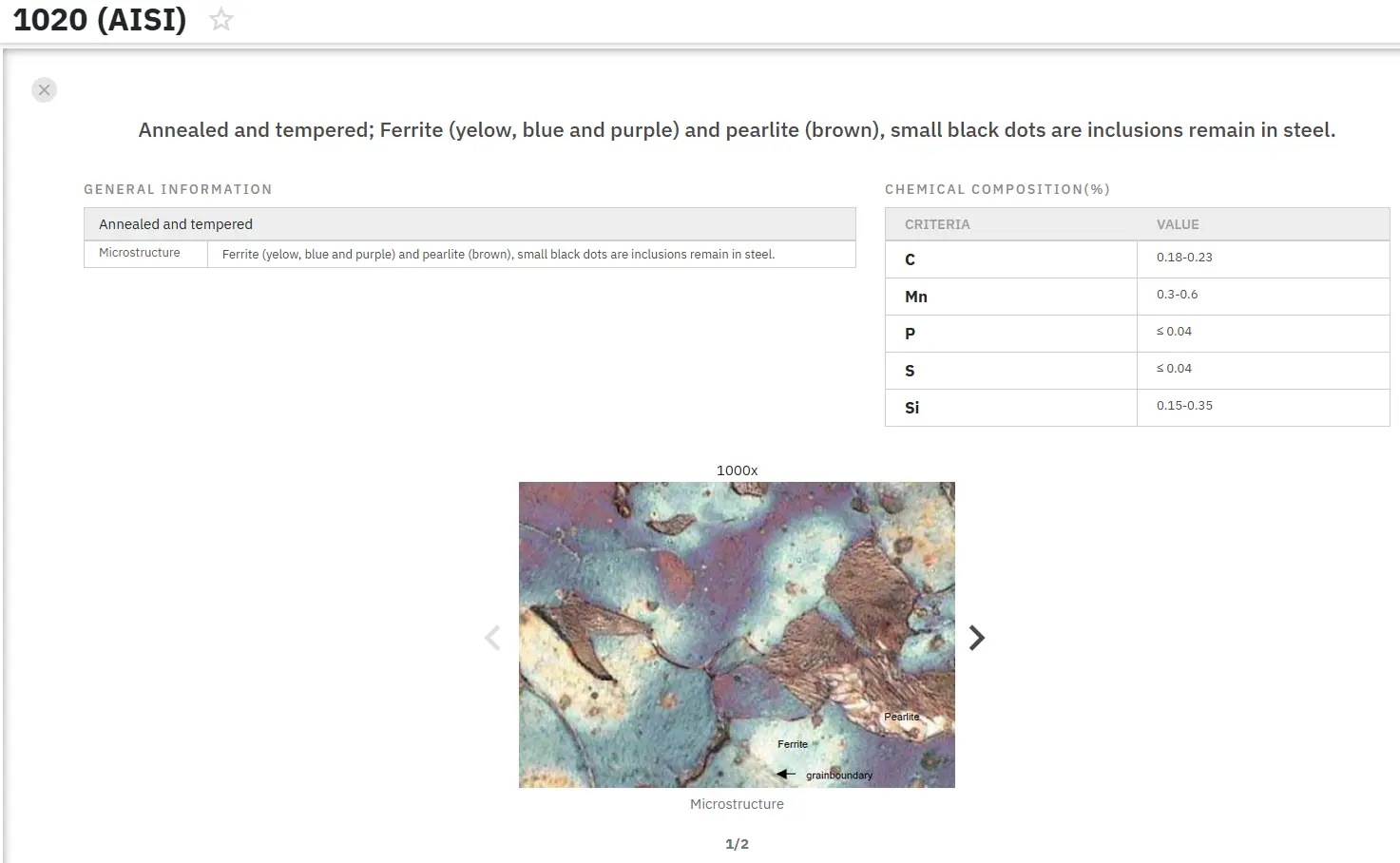
Manganese Sulphide Inclusions in Steel: Part One
Abstract
Non-metallic inclusions, particularly manganese sulphide (MnS), significantly influence steel properties with dual effects. While sulphur enhances machinability, it simultaneously compromises critical service properties including forgeability, ductility, toughness, weldability, and corrosion resistance. This article explores how MnS inclusions form during solidification, their classification into three distinct morphological types, and their impact on mechanical properties. Understanding these inclusions is crucial for optimizing steel performance across various industrial applications and implementing effective cleanliness techniques to mitigate their negative effects while preserving beneficial properties.
Introduction to Non-metallic Inclusions
Non-metallic inclusions represent an ongoing challenge in steel production, adversely affecting mechanical properties and overall material performance. These inclusions create discontinuities within the metallic matrix, reducing the effective cross-sectional area and acting as stress concentrators. Their presence limits dislocation mobility, initiates cracks, and significantly diminishes multiple performance characteristics including strength, plasticity, toughness, fatigue resistance, corrosion resistance, wear properties, and weldability.
The negative impact varies according to inclusion location and size. Intercrystalline inclusions, particularly those that are coarse and present in high concentrations, cause the most severe property degradation. The matrix-inclusion relationship also influences performance—plastic inclusions maintain better adhesion with the surrounding matrix, while hard inclusions, especially rough oxides, create localized stress concentrations that promote crack formation.
Propagation Mechanics and Material Properties
Crack propagation dynamics are directly influenced by inclusion characteristics. Brittle inclusions can fracture under stress fields, creating secondary cracks that accelerate overall crack propagation. Conversely, hard inclusions that maintain adhesion to the matrix can actually reduce crack propagation velocity by providing resistance to crack advancement.
The fundamental challenge stems from the significant thermal and mechanical property differences between non-metallic inclusions and the metal matrix. These disparities generate stresses, promote crack formation, induce creep, create microstructure instability, and cause numerous other detrimental effects during both thermomechanical processing and service loading of steels.
Steel Cleanliness Techniques
The pursuit of inclusion-free steel has driven continuous efforts in both academic research and industrial practice. Several clean steel fabrication techniques have been implemented in large-scale production, including electromagnetic stirring, bubbling, and filtration. However, these conventional methods demonstrate limited effectiveness when targeting particles smaller than 20 μm and typically involve considerable energy consumption, presenting opportunities for further technological advancement.
Sulphur in Steel: Dual Effects
Sulphur presents a dichotomy in steel production. While it substantially improves machinability, it simultaneously produces harmful effects on crucial service properties including forgeability, ductility, toughness, weldability, and corrosion resistance. This contradiction requires careful management in steel composition.
The solid solubility of sulphur in iron (within the Fe-S system) at temperatures below 769°C is extremely limited—less than 0.01% at room temperature. Consequently, sulphur typically exists in steel as sulphide inclusions, predominantly as manganese sulphide (MnS), which form during solidification.
Manganese Sulphide Inclusion Properties
MnS inclusions possess a high melting temperature of 1610°C and form as primary idiomorphic crystals during solidification. While they adversely impact mechanical properties, physical characteristics, and corrosion resistance, they also offer beneficial effects by improving machinability and inhibiting grain growth in steels.
The morphology of MnS inclusions significantly influences various steel properties. According to the classification established by Sims and Dhale, MnS inclusions can be categorized into three distinct types:
- Type 1: Randomly dispersed globular sulphides
- Type 2: Grain boundary sulphides
- Type 3: Angular sulphides

Figure 1: Distribution of the MnS inclusions at different temperatures (Etching: KLEMM Na2S2O3-K2SO4 x200
En savoir plus
Accédez en quelques instants à des milliers d’images de microstructure !
Total Materia Horizon contient une collection unique d’images de métallographie pour une vaste gamme d’alliages métalliques avec leurs états métallurgiques, issus de différents pays et normes.
Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.