Corrosion and Corrosion Properties of Stainless Steels: Part Three
Abstract
Intergranular corrosion, also called intercrystalline corrosion, occurs on or adjacent to the grain boundaries of a metal. It is caused by microsegregation of impurities and alloying elements on the grain boundaries.
The driving force of intergranular corrosion is the difference between the electrode potentials of the grain boundary and the grain itself, which form a galvanic cell in presence of an electrolyte.
Intergranular corrosion of stainless steels
The microstructure of metals and alloys consists of a granular composition. Grains are small crystals whose surfaces join the surfaces of other grains to form grain boundaries. Grain boundaries separate the grains. Intergranular corrosion, also called intercrystalline corrosion, occurs on or adjacent to the grain boundaries of a metal. Some causes of intergranular corrosion are welding, stress annealing, improper heat treating or overheating in service. Inspectors have difficulty in detecting the early stages of intergranular corrosion. This form of corrosion results in a loss of strength in metal parts where the grains have fallen out.
Intergranular corrosion is caused by microsegregation of impurities and alloying elements on the grain boundaries. The driving force of intergranular corrosion is the difference between the electrode potentials of the grain boundary and the grain itself, which form a galvanic cell in presence of an electrolyte.
If the phases segregated at the grain boundaries have lower value of electrode potential they will oxidize (anodic reaction) and the grain metal having higher value of electrode potential will provide cathodic reaction (reduction). Dissolution of anodic grain boundaries starts from the surface and advances along the grains interfaces. The process results in deterioration of the bonding between the grains and drop of mechanical properties.
If the precipitates at the grain boundaries have higher electrode potential the grains will dissolve (anodic reaction). In this case the grain boundaries will not be attacked. Figure 1 shows the intergranular corrosion.

Figure 1: Intergranular corrosion.
Stainless steel has a very thin and stable oxide film rich in chrome. This film reforms rapidly by reaction with the atmosphere if damaged. If stainless steel is not adequately protected from the atmosphere during welding or is subject to very heavy grinding operations, a very thick oxide layer will form. This thick oxide layer, distinguished by its blue tint, will have a chrome-depleted layer under it, which will impair corrosion resistance. Both the oxide film and depleted layer must be removed, either mechanically (grinding with a fine grit is recommended, wire brushing and shot blasting will have less effect), or chemically (acid pickle with a mixture of nitric and hydrofluoric acid). Once cleaned, the surface can be chemically passivated to enhance corrosion resistance, (passivation reduces the anodic reaction involved in the corrosion process).
Carbon steel tools, also supports or even sparks from grinding carbon steel, can embed fragments into the surface of the stainless steel. These fragments can then rust if moistened. Therefore it is recommended that stainless steel fabrication be carried out in a separate designated area and special stainless steel tools used where possible.
If any part of stainless-steel is heated in the range 900-1400°F (482-760°C) for any reasonable time there is a risk that the chrome will form chrome carbides Cr23C6 with any carbon present in the steel along the austenite grains. This causes depletion of chromium from the austenitic grains resulting in decreasing the corrosion protective passive film.
This effect is called sensitization. It is also called weld decay since it usually happens during welding process when the zone around the weld is heated.
Figure 2 shows migration of chromium during heating of stainless steels.
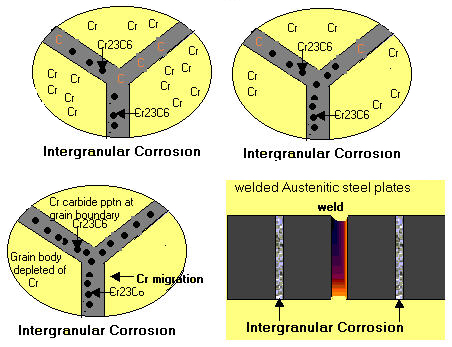
Figure 2: Migration of chromium during heating of stainless steels.
To ensure good corrosion resistance of the weld root, it must be protected from the atmosphere by an inert gas shield during welding and subsequent cooling. The gas shield should be contained around the root of the weld by a suitable dam, which must permit a continuous gas flow through the area.
Welding should not commence until sufficient time has elapsed to allow the volume of purging gas flowing through the dam to equal at least the 6 times the volume contained in the dam. Once purging is complete, the purge flow rate should be reduced so that it only exerts a small positive pressure, sufficient to exclude air. If good corrosion resistance of the root is required, the oxygen level in the dam should not exceed 0.1% (1000 ppm); for extreme corrosion resistance this should be reduced to 0.015% (150 ppm).
Backing gasses are typically argon or helium; nitrogen is often used as an economic alternative where corrosion resistance is not critical, nitrogen + 10% helium is better. A wide variety of proprietary pastes and backing materials are available than can be use to protect the root instead of a gas shield. In some applications where corrosion and oxide coking of the weld root is not important, such as large stainless steel ducting, no gas backing is used.
Figure 3 shows two microstructures of type 304 stainless steel. The figure on the left is the normalized microstructure and the one on the right is the "sensitized" structure and is susceptible to intergranular corrosion or intergranular stress corrosion cracking.
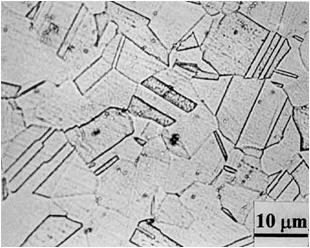
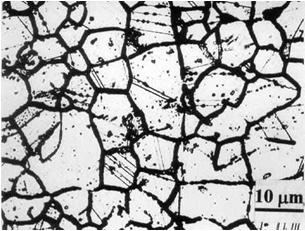
Figure 3: Microstructure of stainless steel type 304.
Means of preventing sensitization:
- Solution heat treatment: heating to a temperature above 1900°F (1040°C) followed by quenching (rapid cooling) in water or quenching oils. During the heating stage the carbides dissolve and their formation is suppressed by fast cooling.
- Lowering concentration of carbon. Sensitization is depressed in low carbon (max. 0.03%) stainless steels, designated with the suffix L (304L, 316L).
- Stabilization by carbide forming elements. Formation of chromium carbides is avoided in stabilized austenitic stainless steels (321, 347) containing carbide forming elements like titanium, niobium, tantalum, zirconium. Stabilization heat treatment of such steels results in preferred formation of carbides of the stabilizing elements instead of chromium carbides.
Accédez immédiatement à des données précises en corrosion !
Total Materia Horizon contient le comportement en corrosion et des informations sur les propriétés pour des centaines de milliers de matériaux, dans plus de 2000 milieux corrosifs.

Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.