Bulk Metallic Glass: Part Two
Abstract
Bulk metallic glass (BMG) represents a revolutionary advancement in metallurgy, fundamentally different from conventional crystalline metals. Unlike traditional metals with crystalline structures, metallic glasses feature amorphous atomic arrangements that eliminate grain boundaries and dislocations. This unique structure provides exceptional properties including high yield strength, superior corrosion resistance, excellent wear resistance, and superplasticity at elevated temperatures. While conventional metals require complex manufacturing processes and exhibit inherent weaknesses due to grain boundaries, bulk metallic glass can be processed like thermoplastics and offers superior mechanical properties. Applications span from medical implants and surgical instruments to sports equipment and electronic casings. The development of additive manufacturing techniques has expanded BMG production possibilities, though thermal cycling challenges remain. This technology promises to transform multiple industries through materials with unprecedented combinations of strength, durability, and processing flexibility.
Introduction to Bulk Metallic Glass Technology
Amorphous metals, commonly known as metallic glass, possess the potential to revolutionize how we utilize metals across numerous industries. The fundamental question that drives research and development in this field centers on understanding what makes metallic glass alloys so exceptionally desirable compared to conventional materials.
Most metals exhibit crystalline structures that form naturally during solidification, but metallic glasses represent a fundamentally different approach to metallurgy. This structural difference gives bulk metallic glass almost extraordinary properties that make these materials highly sought after across diverse industries and applications.
Understanding Conventional Metal Limitations
Conventional metal alloys demonstrate inherent limitations that stem from their crystalline nature. During cooling from the liquid state, atoms in ordinary alloys arrange themselves into repeating patterns of crystals or grains with varying sizes and shapes. Since metals typically do not solidify into single crystals, they possess inherent structural weaknesses that limit their performance potential.
The boundaries between individual grains represent the primary weak spots in conventional metals. Under sufficient stress or elevated temperatures, these grains slide past each other, resulting in material deformation. Additionally, extra atoms frequently exist within the grain structures, creating planes of distortion called dislocations. These dislocations move easily through metals under stress, again causing deformation and reducing overall material strength.
Grain boundaries and dislocations significantly reduce a metal's strength compared to its theoretical maximum potential. This fundamental limitation has driven researchers to explore alternative approaches to metal structure and processing.
Casting conventional metals requires more manufacturing steps than bulk metallic glass production. Traditional metals shrink significantly during cooling in molds as they transition from liquid to solid form, often developing surface roughness that requires correction. Secondary processing steps such as grinding and polishing are typically necessary to achieve final product specifications, adding cost and complexity to manufacturing processes.
Revolutionary Properties of Bulk Metallic Glass
Bulk metallic glasses exhibit an amorphous structure that provides highly desirable mechanical, magnetic, and corrosion properties. These materials demonstrate high yield stress, low magnetic losses, and exceptional corrosion resistance that surpasses conventional crystalline metals.
Glass-forming alloys have the potential to become amorphous provided the solidification rate remains rapid enough to avoid crystallization during cooling. This critical requirement has historically limited the size and complexity of components that could be produced using traditional manufacturing techniques.
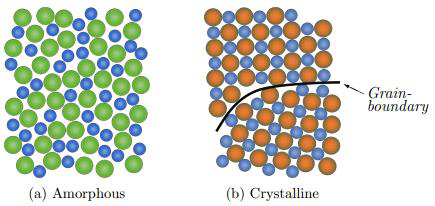
Figure 1: Illustration of the atomic structure of a binary alloy in the crystalline (b) and amorphous (a) state
Traditional manufacturing techniques such as casting limit cooling rates and component sizes due to relatively slow heat conduction in bulk materials. However, additive manufacturing presents new opportunities for bulk metallic glass production. The extremely small melt pools achievable in additive manufacturing enable very rapid solidification that can bypass crystallization entirely.
Despite these advantages, additive manufacturing of bulk metallic glass faces challenges from thermal cycling effects. Crystals may form through devitrification, which occurs when crystal formation is caused by heating of the amorphous phase as new layers are added over previously deposited layers. Process simulation continues to improve understanding of glass formation during additive manufacturing and aids in developing process parameters that control devitrification levels.
Structural Advantages of Metallic Glass
The atomic structure of metallic glass differs dramatically from conventional metals, providing the foundation for its superior properties. Metallic glasses are non-crystalline solid materials with amorphous structures that contrast sharply with traditional crystalline solids.
In metallic glass, atoms are not arranged in periodic lattice structures and appear randomly distributed throughout the material. This lack of ordered orientation means metallic glasses contain no grains or grain boundaries, eliminating the primary weakness points found in conventional metals.
Since grain boundaries make crystalline materials susceptible to corrosion and wear, their absence in metallic glasses results in significantly higher yield strength and superior resistance to both corrosion and wear compared to crystalline counterparts. These properties open numerous application possibilities, with medical equipment and implants representing particularly important areas.
Surgical blades manufactured from metallic glass can achieve extreme sharpness while maintaining long-lasting performance. Microscopic geared engines for advanced medical equipment and micro-factories can be produced at very small scales while providing incredible wear resistance that extends operational life far beyond conventional materials.
Historical Development of Amorphous Metals
The development of amorphous metals began in 1960 when Duwes and colleagues synthesized the first amorphous metal by rapidly solidifying a gold-silicon alloy. Their technique involved shooting small droplets of molten metal onto a cold copper plate using a specialized gun technique. The cooling rate achieved was estimated at approximately 10⁶ K/s, producing splatter with thickness around 50 µm.
The first iron-based metallic glass was synthesized in 1976 by Davis, consisting of 80% iron and 20% boron formed into thin ribbons. During this early period of amorphous metal development, at least one dimension of samples was limited to very small sizes due to the extreme cooling rates required to prevent crystallization.
Heat extraction from molten metal is limited by conduction within the material, meaning large components would experience insufficient cooling rates to maintain amorphous structure. The scientific drive to synthesize thicker samples eventually led to the development of bulk metallic glasses that could achieve substantial dimensions while maintaining their amorphous properties.
Exceptional Properties and Performance Characteristics
Bulk metallic glass exhibits a unique combination of properties that distinguishes it from all conventional materials. High hardness and exceptional wear resistance make these materials ideal for applications requiring long-term durability under demanding conditions. Excellent corrosion resistance extends service life in harsh environments where conventional metals would rapidly degrade.
The high strength-to-weight ratio of bulk metallic glass provides structural advantages in applications where weight reduction is critical. Superior elastic limit performance allows these materials to undergo significant deformation without permanent damage, returning to original shape when stress is removed.
Superplasticity at high temperatures enables complex forming operations that would be impossible with conventional metals. Additionally, bulk metallic glass demonstrates excellent soft magnetic properties that make it valuable for electrical and electronic applications requiring precise magnetic characteristics.
Diverse Applications Across Industries
The unique properties of bulk metallic glass have enabled applications across numerous industries. Thermoplastic forming capabilities allow metals to be processed similarly to polymers, opening entirely new manufacturing approaches that combine the strength of metals with the processing flexibility of plastics.
Sports equipment manufacturers utilize bulk metallic glass to create products with superior performance characteristics. The combination of high strength, low weight, and excellent durability makes these materials particularly attractive for golf clubs, tennis rackets, and other sporting goods where performance advantages translate directly to user benefits.
Sensors and actuators benefit from the precise mechanical properties and corrosion resistance of bulk metallic glass. Electronic casings manufactured from these materials provide superior protection while maintaining excellent electromagnetic properties. Industrial coatings applications leverage the corrosion resistance and wear properties to extend equipment life in challenging environments.
Table 1. Specific examples of bulk metallic glass compositions that demonstrate the variety of alloy systems available for different applications
| Alloy | Zr (%) | Cu (%) | Ni (%) | Ti (%) | Be (%) | Al (%) |
Other Elements |
| Vit1b | 67.0 | 10.6 | 9.80 | 8.80 | 3.80 | - | - |
| Vit601 | 62.5 | 31.0 | 3.20 | - | 0.10 | 3.30 | - |
| Vit105 | 65.7 | 15.6 | 11.8 | 3.30 | - | 3.70 | - |
| Vit106a | 70.1 | 13.0 | 9.90 | - | - | 3.60 | Nb 3.40 |
| GMT | - | 8.50 | 76.0 | 9.40 | - | - | Nb 5,20 Pb 9.40 Si 0.30 B 0.60 |
| Pt850 | - | 7.10 | 2.36 | - | - | - | Pt 85,24 P 5.30 |
| JPL | - | 7.00 | - | 43.00 | - | 3.00 | B 6.00 |
Table 2. Quantitative property data that illustrates the superior performance characteristics of various bulk metallic glass compositions
| Parameter | Units | Vit1b | Vit601 | Vit105 | Vit106a |
| Yield Strength | MPa | 1800 | 1795 | 1850 | 1800 |
| Elastic Modulus | GPa | 95 | 91 | 95 | |
| Fracture Toughness | MPa√m | 55 | 70 | 75 | 30 |
| Density | g/cc | 6.0 | 6.9 | 6.6 | 6.7 |
| Glass Transition (Tg) | °C | 352 | 420 | 403 | 395 |
| Crystallization (Tx) | °C | 466 | 495 | 469 | 499 |
| Melt Temperature | °C | 644 | 753 | 805 | 837 |
Commercial Success and Real-World Applications
The commercial viability of bulk metallic glass has been demonstrated through successful production of numerous components across different industries. These achievements showcase the material's transition from laboratory curiosity to practical engineering solution.

Figure 2: Examples of bulk metallic glass hardware, including golf clubs, electronic casings, optical hardware, ingots, 12 mm diameter rod, and large plate
Commercial applications include golf club drivers that provide superior performance through optimized weight distribution and enhanced durability. Electronic device casings benefit from the combination of strength, corrosion resistance, and precise dimensional control achievable with bulk metallic glass. Optical hardware applications leverage the smooth surface finish and dimensional stability that these materials provide.
The production of large plates measuring approximately 1 m² demonstrates the scalability of bulk metallic glass manufacturing processes. This capability opens possibilities for architectural and structural applications where the unique properties of these materials can provide significant advantages over conventional options.
Future Prospects and Technological Advancement
The continued development of bulk metallic glass technology promises to expand applications across numerous industries. Advances in additive manufacturing techniques are addressing the thermal cycling challenges that have limited component complexity and size. Improved process control and simulation capabilities are enabling more precise control over material properties and reducing defect rates.
Research into new alloy compositions continues to expand the range of properties available, targeting specific applications that require unique combinations of characteristics. The development of processing techniques that can produce larger components while maintaining amorphous structure will enable new applications in aerospace, automotive, and construction industries.
The integration of bulk metallic glass with other advanced materials and manufacturing processes represents an exciting frontier that could revolutionize how we approach engineering design and materials selection. As production costs decrease and manufacturing capabilities expand, bulk metallic glass is positioned to become a mainstream engineering material that transforms multiple industries through its unprecedented combination of properties and processing flexibility.
En savoir plus
Accédez en quelques instants aux compositions précises des matériaux !
Total Materia Horizon contient les compositions chimiques de centaines de milliers de matériaux, ainsi que leurs propriétés mécaniques et physiques, et bien plus.
Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.