Alloy steels
Abstract
During the last fifty years engineers have demanded steels with higher and higher tensile strength, together with adequate ductility. This has been particularly so where lightness is desirable, as in the automobile and aircraft industries. An increase in carbon content met this demand in a limited way, but even in the heat-treated condition the maximum strength is about 700 MPa above which value a rapid fall in ductility and impact strength occurs and mass effects limit the permissible section.
During the last fifty years engineers have demanded steels with higher and higher tensile strength, together with adequate ductility. This has been particularly so where lightness is desirable, as in the automobile and aircraft industries. An increase in carbon content met this demand in a limited way, but even in the heat-treated condition the maximum strength is about 700 MPa above which value a rapid fall in ductility and impact strength occurs and mass effects limit the permissible section.
Heattreated alloy steels provide high strength, high yield point, combined with appreciable ductility even in large sections. The use of plain carbon steels frequently necessitates water quenching accompanied by the danger of distortion and cracking, and even so only thin sections can be hardened throughout. For resisting corrosion and oxidation at elevated temperatures, alloy steels are essential.
The Alloy Steels Research Committee adopted the following definition: “Carbon steels are regarded as steels containing not more than 0,5% manganese and 0,5% silicon, all other steels being regarded as alloy steels”.
The principal alloying elements added to steel in widely varying amounts either singly or in complex mixtures are nickel, chromium, manganese, molybdenum, vanadium, silicon and cobalt.
The effect of the alloying element in the steel may be one or more of the following:
(1) It may go into solid solution in the iron, enhancing the strength. The general effectiveness is shown in Fig. 1.
(2) Hard carbides associated with Fe,C may be formed.
(3) It may form intermediate compounds with iron, e.g. FeCr (sigma phase), Fe,W,.
(4) It may influence the critical range in one or more of the following ways:
(a) Alter the temperature. For example, 3% nickel lowers the Ac points some 30°C, while 12% chromium raises the Ac1, temperature to about 800°C and also forms a range of 150/200°C above this in which the pearlite changes to austenite. Fig. 2 shows the effect of alloys on the eutectoid temperature.
(b) Alter the carbon content of the eutectoid (Fig. 2). The carbon content of the pearlite in a 12% chromium steel is 0,33%, as compared with 0,87 in an ordinary steel. Nickel also reduces the amount of carbon in the pearlite and consequently increases the volume of this constituent at the expense of the weaker ferrite.
(c) Alter the “critical cooling velocity”, which is the minimum cooling speed which will produce bainite or martensite from austenite. Typical critical speeds obtained by quenching from 950°C are given in Table 1.
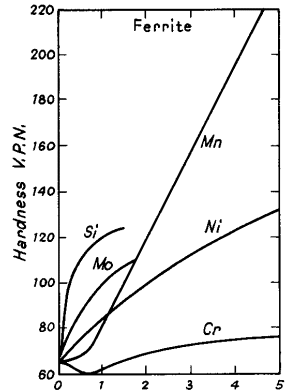
Figure 1. Hardening effects of alloying elements in solid solution in fully annealed ferrite (Austin)
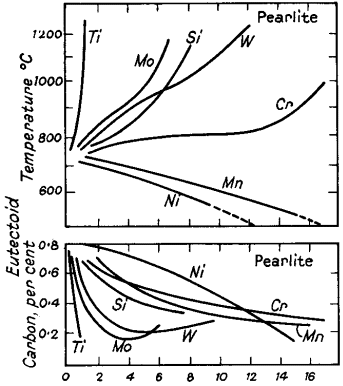
Figure 2. Effects of alloying elements on the carbon content and temperature of the eutectoid point (Bain)
| Carbon, % | Alloying Element, % | Cooling Speed to form Martensite, °C per sec (650°C) |
| 0.42 | 0.55 Mn | 550 |
| 0.40 | 1.60 Mn | 50 |
| 0.42 | 1.12 Ni | 450 |
| 0.40 | 4.80 Ni | 85 |
| 0.38 | 2.64 Cr | 10 |
Table 1. Effect of alloying on the critical cooling speed of steel
The efficiency of the additions of the various alloy elements in reducing the effect of mass during quenching may be judged by the relative reduction of the critical velocity of the steel. Chromium and manganese respectively are far more effective than nickel.
(5) Combinations of elements can be chosen so that the volume change is reduced and also the risk of quench cracking. It may produce effeets characteristic of the alloying element.
(a) It may render the alloy sluggish to thermal changes, increasing the stability of the hardened condition and so producing tool steels which are capable of being used up to 550°C without softening and in certain cases may exhibit an increase in hardness.
(b) It may have a chemical effect on the impurities. Under suitable slag conditions vanadium, in quite small quantities, "cleans" the steel and renders it free from slag inclusions. Manganese and zirconium form sulphides.
(c) Certain elements such as chromium, Aluminium, silicon and copper tend to produce adherent oxide films on the surface of the steel which increase its resistance to corrosion and oxidation at elevated temperatures.
(d) Creep strength may be increased by the presence of a dispersion of fine carbides, e.g. molybdenum.
Classification of alloying additions
Classification of alloying metals according to their effect in the steel is difficult, because the influence varies so widely with each addition depending on the quantity used and other elements present. A useful grouping, however, is based upon the effect of the element on (a) the stability of the carbides and (b) the stability of the austenite.
(1) Elements which tend to form carbides. Chromium,tungsten,titanium, columbium, vanadium, molybdenum and manganese. The mixture of complex carbides is often referred to as cementite.
(2) Elements which tend to graphitise the carbide. Silicon, cobalt, aluminium and nickel. Only a small proportion of these elements can be added to the steel before graphite forms during processing, with attendant ruin of the properties of the steel, unless elements from group 1 are added to counteract the effect.
(3) Elements which tend to stabilise austenite. Manganese, nickel, cobalt and copper.
These elements alter the critical points of iron in a similar way to carbon by raising the A4 point and lowering the A3 point, thus increasing the range in which austenite is stable, and they also tend to retard the separation of carbides. They have a crystal lattice (f.c.c.) similar to that of g-iron in which they are more soluble than in a-iron.
(4) Elements which tend to stabilise ferrite. Chromium, tungsten, molybdenum, vanadium and silicon .
These elements are more soluble in a-iron than in g-iron. They diminish the amount of carbon soluble in the austenite and thus tend to increase the volume of free carbide in the steel for a given carbon content. On the binary equilibrium diagram of these elements with pure iron the A4 point is lowered and A3 raised (although it may be lowered initially), until the two points merge to form a “closed gamma loop”.
Thus, with above, a certain amount of each of these elements the austenite phase disappears and ferrite exists from the melting-point down to room temperature. No critical points exist and such steels (e.g. 18% chromium irons) are not amenable to normal heat treatment, except recrystallisation after cold work. This effect, however, can be counteracted by adding elements from group 3. For example, 2% of nickel is added to the 18% chromium stainless steel to enable it to be refined by normal heat-treatment; carbon has the same effect. Aluminium has the reverse effect in 12 % chromium steel.
Accédez immédiatement à des propriétés précises sur les aciers de construction !
Total Materia Horizon contient les propriétés de plus de 150.000 aciers de construction : composition, propriétés mécaniques et physiques, propriétés non-linéaires et bien plus.
Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.