The Origins of Oxide Inclusions
Abstract
Generally, inclusions degrade the mechanical properties of the steel by decreasing the toughness of the cast metal and increasing the risk for mechanical and/or corrosive failure of the final product.
While trying to maintain equilibrium with the surroundings, inclusions may be undercooled during some steps of the treatment and result in amorphous phases, or solidify and take the form of supersaturated solid solution.
The presence of non-metalic oxide inclusions is a major concern in many grades of commercial steel. Generally, inclusions degrade the mechanical properties of the steel by decreasing the toughness of the cast metal and increasing the risk for mechanical and/or corrosive failure of the final product. Oxide inclusions are usually classified into two categories, (depending on their origin): (i) as residual products resulting from intentionally added alloying elements for de-oxidation in the ladle and (ii) as reduction products resulting from reactions between the melt and the atmosphere, slag or refractory. Examples of the latter (often referred to as exogenous or macro-inclusions) are the results of re-oxidation of the molten metal or slag emulsification in the liquid.
Details of the formation or origin of exogenous inclusions are summarized below:
- Reoxidation of deoxidized and refined steel melt at the surface of the teeming stream by air ingress from the joints at the ladle/long nozzle, tundish/slide gate. And slide gate/submerged entry nozzle;
- Reoxidation of the melt bath surface in tundish by air ingress;
- Reoxidation of the melt bath surface by iron oxide, manganese oxide, and/ or silica that are contained either in carried over ladle slag, flux added to tundish, or a mixture of the two;
- Entrainment of the ladle slag into melt in the tundish by vortexing/draining near the end of the emptying of the ladle;
- Emulsification and entrainment of tundish slag floating on the melt surface by turbulent impinging teeming stream from the ladle during the opening and changing of the ladle;
- Entrainment of tundish slag floating on the melt surface by turbulent surface flow or by vortexing towards the outlet of the tundish, particularly when the bath depth in the tundish is decreased to a low level during the ladle change period;
- Reoxidation of the melt at the melt/refractory interface by less stable oxides contained in tundish glaze, the nozzle, and/or the tundish lining;
- Entrainment of the fragments of the ladle glaze, ladle nozzle, long nozzle, tundish lining, and/or submerged entry nozzle due to erosion and detachment by turbulent melt flow;
- Entrainment of alumina clusters, which are deposited on the inner wall of the tundish nozzle and the SEN and dislodged by turbulent melt flow to the mold;
Indigenous inclusions, such as deoxidation products, are generated by chemical reactions between dissolved species in the steel bath and are generally smaller in size. Deoxidation products originate from the reaction between dissolved oxygen and added deoxidants and can be both solid and liquid at steelmaking temperatures. The presence of a few large indigenous inclusions has a strong effect on the properties of steel products.
Indigenous inclusions often go through a series of transformations as the steel cools from 1600°C to room temperature. While trying to maintain equilibrium with the surroundings, inclusions may be undercooled during some steps of the treatment and result in amorphous phases, or solidify and take the form of supersaturated solid solution. Indigenous inclusions can therefore categorized into formation steps, as summarized below:
I. Primary inclusions: generated during deoxidation reaction
II. Secondary inclusions: generated due to equilibrium shift as temperature decreases during vessel transfer, such as tapping and teeming operations
III. Tertiary inclusions: generated during the process of solidification, usually characterized by rapid cooling
IV. Quaternary inclusions: generated during solid state phase transformation, which causes changes in solubility limits of various constituents.
Deoxidized melt is commonly refined in the ladle to trim temperature and chemical composition and to remove deoxidation products. Ladle refining usually removes most of the large indigenous inclusions, leaving only a small amount of inclusions of up to 20-50 µm in diameter suspended in the melt. Inclusions greater than about 50 µm, often of exogenous origin, may be called macro inclusions. Inclusions smaller than about 50 µm, mostly of indigenous origin, and small agglomerates of the indigenous inclusions may be called micro inclusions.
The above definitions are not unique, but are used for convenience.
Inclusions in the cast strands greater than the critical size impair the properties of steel products are listed in Table 1.
| Steel products | Critical size (µm) in slabs and blooms |
| Cold rolled sheet | 240 |
| DI-can | 50 |
| UOE-pipe | 200 |
| ERW-pie | 140 |
| Cold forgings | 100 |
| Steel cord | 30 |
| Ball bearing | 15 |
| Typical composition | Al2O3, CaO-(MgO)-Al2O3, CaO-Na2O-SiO2-Al2O3, CaO-MgO-SiO2-Al2O3 |
Table 1: Critical size of macro inclusions in cast semis that cause process upsets and product defects
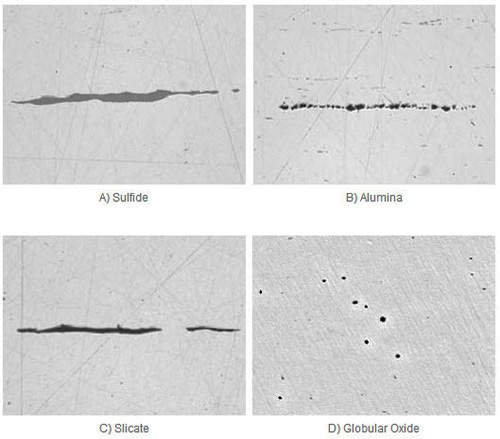
Figure 1: Example of sulfide, alumina, silicate and glopbular oxide in steel
¡Encuentre al Instante Miles de Diagramas de Metalografía!
Total Materia Horizon contiene una colección única de imágenes de metalografía de una amplia gama de aleaciones metálicas, países, normas y tratamientos térmicos.
Obtenga una cuenta de prueba GRATUITA de Total Materia Horizon y únase a nuestra comunidad que traspasa los 500.000 usuarios provenientes de más de 120 países.