Overview of Spinodal Decomposition of Non-Ferrous Alloys: Part Two
Abstract
Spinodal decomposition manifests itself as characteristic patterns in an alloy, which are a direct result of phase separation during the quenching process.
General spinodal decomposition properties and benefits include, to name but a few, excellent high temperature stress relaxation resistance, lack of distortion during aging, lower initial cost and cost savings during processing.
The addition of Ni between 4 and 15% and Sn between 4 and 8% to the Cu matrix constitutes spinodally decomposable Cu-Ni-Sn alloy system. The spinodally decomposable Cu-Ni-Sn Bronze alloys produce a modulated microstructure during the heat treatment process and its mechanical properties comparable to those of Cu-Be alloys, while being relatively inexpensive and hazard free. The modulated microstructure significantly increases the strength of the Cu-Ni-Sn alloys, and the increase is attributed to a) alloy composition (b) condition of the alloy cast or wrought) (c) amount of cold work prior to aging d) aging temperature and e) aging time. The Cu-Ni-Sn Bronze alloys can be used as the friction-reducing and anti-wear materials to make high performance bearings for aerospace, roller cone rock bit and heavy duty mobile industrial equipment etc.
It has been generally observed that five different transformation products exist in the Cu-Ni-Sn system as shown in Figure 1. They are: a) modulated structure resulting from spinodal decomposition b) DO22 ordered structure c) L12 ordered structure d) grain boundary and intra-granular γ DO3) precipitates and e) discontinuous γ precipitates. The above transformation is dependent on temperature and time Refer to Figure 2). At high temperature the grain boundary and intergranular γ DO3) precipitates form, whereas, in the middle range of temperature a discontinuous γ precipitates forms. Below the critical temperature TR ~457°C. the spinodal decomposition takes place. When the aging time is increased, an ordering reaction takes place forming DO22 and L12 ordered structure (Refer to Figure 2). The spinodal decomposition and ordering reaction increases the hardness and the YS of the alloys.
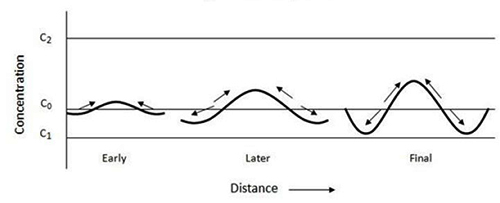
Figure 1: Spinodal decomposition process
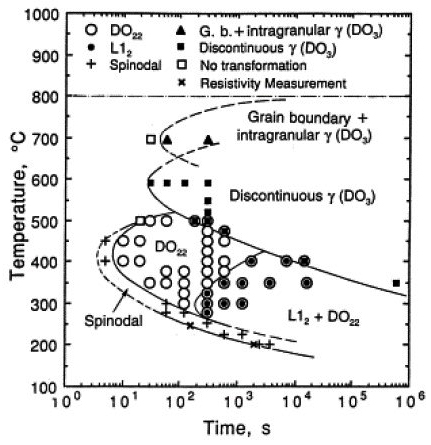
Figure 2: A typical phase transformation diagram for the Cu-Ni-Sn Bronze alloy
Spinodal decomposition properties and benefits
- Excellent high temperature stress relaxation resistance
- High strength and excellent formability
- Lack of distortion during aging
- Available in mill hardened and age hardenable tempers
- Excellent solderability and resistance to intermetallic formation at high temperature
- Excellent corrosion resistance and ease of cleaning. In moist ammonia it resists corrosion for over 500 hrs. at 400°C
- Lower initial cost and cost savings during processing
Limitations of spinodal decomposition
1. Limited alloying compositions are suitable for spinodal hardening.
2. It requires homogenization, solution treatment and aging treatment i.e. it is time consuming.
3. In ternary alloys metastable phase is of very short moment, so special care should be taken to achieve this region.
4. Micro examinations are needed in every stage of experiment.
5. Behavior of Spinodal decomposition may sometimes unpredictable.
Applications
1. Marine Environment
2. Automotive
3. Offshore oil and gas
4. Aerospace
¡Encuentre al Instante Miles de Diagramas de Tratamiento Térmico!
Total Materia Horizon contiene detalles de tratamiento térmico para cientos de miles de materiales, diagramas de templabilidad, templado de dureza, diagramas TTT y CCT, y mucho más.

Obtenga una cuenta de prueba GRATUITA de Total Materia Horizon y únase a nuestra comunidad que traspasa los 500.000 usuarios provenientes de más de 120 países.