Continuous Electroplating Process for Steel Sheet Products
The steel sheet electroplating process utilizes the same basic principle as that for conventional decorative finish electroplating. However, the steel sheet process differs in that the electroplated coating is applied by passing the strip at high speeds through a series of plating cells, building the coating thickness by a small amount each time the strip passes through an individual cell.
Introduction
The development of modern continuous sheet galvanizing lines has led to the disappearance of most of the old manual mills for galvanizing cut sheets. There are however still some machines that galvanize cut-to-length sheets; they use chemical pretreatment sequence similar to those for wire or tube galvanizing.
At the beginning of the line, the end of one coil is welded to the start of the next coil. Then there are two basic methods for continuously galvanizing sheet which differ in the way that the strip is cleaned before galvanizing-chemically or by thermal treatments. Coils of annealed cold reduced sheet may be fed directly to the galvanizing line, or alternatively, coiled sheet is continuously heat treated in the pretreatment line. After leaving the galvanizing bath, in which strip only stays for a few seconds, the surface is wiped to remove excess zinc and may be further treated to after the surface appearance, composition, smoothness or mechanical properties.
The steel sheet electroplating process utilizes the same basic principle as that for conventional decorative finish electroplating. However, the steel sheet process differs in that the electroplated coating is applied by passing the strip at high speeds through a series of plating cells, building the coating thickness by a small amount each time the strip passes through an individual cell. This continuous process for electroplating steel strip requires necessary equipment to transport the strip at high speeds (150 to 200 meters per minute and higher) through a series of individual plating cells, and is not as simple as it sounds.
An Electroplating Cell
The simplest electroplating cell is shown in the sketch where the plating solution bath is zinc sulfate.
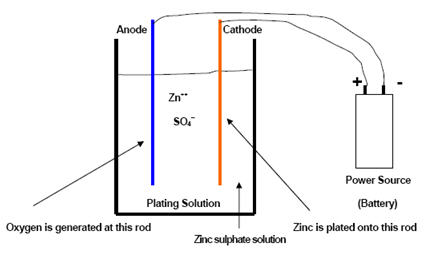
Figure 1: The common schema of the electroplating cell.
This simple plating cell illustrates the actions during the plating process. At cathode (steel, for example), zinc ions dissolved in the zinc sulfate solution combine with two electrons and form elemental zinc, which deposits onto the cathode surface. At anode, water is converted to oxygen and hydrogen ions to maintain electrical balance. The oxygen forms a gas and nothing is deposited on the anode surface. The plating solution carries the current between the cathode and anode.
Plating of Steel Sheet in a Continuous Process
There are many types of anode arrangements. Some are horizontal, others are vertical, and one process utilizes a radial cell wherein the strip passes around large diameter rolls inside each plating cell, and the anodes have a radial design to match the diameter of the large rolls submerged into the plating solution. Each type of anode arrangement and design has advantages and disadvantages; thus, it is easy to see why different manufacturers use different methods. Each requires very close control of the anode-to-strip spacing to achieve efficient plating, avoiding arc spots and other defects in the coating.

Figure 2: Modern Continuous Electroplating Line.
Maintenance of the large volume of plating solution that is contained in all the cells is a science unto itself. Whether the plating solution for electrogalvanizing is based on zinc sulfate or zinc chloride chemistry, maintenance of the proper ranges of zinc ion concentration and solution pH are important control features. Besides plating zinc, some manufacturers have the ability to deposit alloy coatings. This requires, at a minimum, at least one more level of control of the plating solution. For example, producing a zinc/nickel alloy coating requires close control of the concentrations of both the dissolved zinc and nickel in the solution. Solution control has to be accomplished on a dynamic basis since these lines operate continuously.
Power Requirements
The electroplating process requires a large amount of electric power to deposit a metallic coating. The total power requirement is a direct function of the coating thickness that is needed to meet the customer’s specification. For example, the power required to deposit a zinc coating mass of 80 g/m2 is approximately twice that required to deposit a coating of 40 g/m2. A typical line that has the capability to process 70 to 120 tons/hour with a coating mass of 50 g/m2 will consume hundreds of thousands of amperes during this one hour of processing time. It is easy to see why power costs are major cost component for a facility that processes large quantities of electroplated sheet product.
Product Types
The most common electroplated coating for steel sheet products is zinc. Electrogalvanized zinc coatings are used by a number of automotive companies for exposed car-body panels, where the typical coating mass ranges from about 50 to 80 g/m2 per side. These coatings are considerably thicker than the electrogalvanized coatings typically used for non-automotive applications, so the lines built to make products for automotive applications usually have a large number of plating cells. Also, each automotive customer has their own specific coated-product specification.
Another attribute associated with the use of electrogalvanized coatings for automotive applications is excellent surface finish that is attainable with the electroplating process. Twenty-five years ago, when automotive companies began using large amounts of galvanized sheet for exposed body panels to improve corrosion protection, one of the few coated sheet products that could meet the demanding surface quality requirements was electrogalvanized. Hot-dip galvanized was, and still is, used for unexposed body parts. As the surface of hot-dip products improves, they continue to replace electrogalvanized sheet for exposed automotive body panels.
Other zinc electroplating lines have been built through the years to make thinner coatings. Typically, the sheet that is made on these lines has a coating mass of less than 25 g/m2. The applications for this product are often indoors; applications where the environment is not very corrosive. Many applications involve painted products. These coating lines often have the ability to apply paint pre-treatment so that the customer can paint directly without additional in-house treating.
A second type of electroplated coated-steel sheet being manufactured today has a coating composed of a zinc/nickel alloy. Typically, the nickel content is 10 to 16 percent with the balance being zinc. The unique feature of this process is that the zinc and nickel ions are co-deposited to make a true alloy coating. It is not composed of alternating layers.
The application for this product has been limited primarily to a few automotive companies. These companies have developed in-house product design and manufacturing processes to take advantage of the unique characteristics of the zinc/nickel coating. For these automotive applications, the metallic coating is often coated with a special corrosion-resistant thin organic coating on top of the zinc/nickel. The zinc/nickel alloy coating is covered by ASTM Specification A 918.
A third type of electroplated coating is zinc/iron alloy coating. The attributes of this specialized coating are somewhat like those of hot-dip galvannealed product. Like zinc/nickel alloy, zinc/iron coating is co-deposited as an alloy coating. Iron is uniformly deposited throughout the coating thickness. Also, like zinc/nickel coating, zinc/iron coating is used predominantly by the automotive industry. The attributes of electroplated zinc/iron is that it is relatively easy to weld and paint if the proper electro-priming equipment is available to the automotive manufacturer. Also, the coating is very hard, making it is less susceptible to scratching during stamping and handling. This is the important feature since the zinc/iron alloy coated-sheet product is being used almost exclusively for exposed car-body panels.
Corrosion Resistance of Electroplated Coatings
Concerning the corrosion behavior of electrogalvanized versus hot-dip galvanized coating, it is important to note that it is essentially equivalent for identical coating masses. A coating mass of 100 g/m2 will provide essentially the same amount of corrosion protection whether it is a hot-dip galvanized or electrogalvanized coating.
The reason that the automotive companies can successfully use a coating mass in the 50 to 80 g/m2 range is because they apply additional treatments on top of the metallic coating, including a zinc phosphate coating, an electro-deposited organic-based coating, a primer, and multiple-layer finishing paint coatings. Clearly, the corrosion resistance needed to protect a car body panel for over 10 years is more than that afforded by the metallic coating alone. Application of the above coatings over the electroplated metallic layer results in a synergistic system, whose corrosion resistance is more than the sum of its individual components.