Wet Corrosion
Abstract
This article provides a comprehensive examination of wet corrosion, a fundamental type of material degradation that occurs through electron transfer in the presence of moisture. The text details the electrochemical mechanisms distinguishing wet corrosion from dry corrosion, exploring the complex interplay of oxidation and reduction processes. Through detailed analysis of environmental factors, chemical reactions, and electrochemical theory, the article explains how wet corrosion affects metallic structures and materials. Special attention is given to the various reaction pathways, equilibrium states, and the factors that influence corrosion rates in different environments.
Introduction to Corrosion Fundamentals
Corrosion is defined as the deterioration of a material through chemical or electrochemical reactions with its environment. This degradation process significantly impacts useful properties of metals, including malleability, ductility, electrical conductivity, and surface appearance. The most familiar example of corrosion is the rusting of iron when exposed to atmospheric conditions.
Two distinct types of corrosion exist: dry corrosion, occurring without the presence of moisture, and wet corrosion, which involves the transfer of electrons during oxidation and reduction processes.
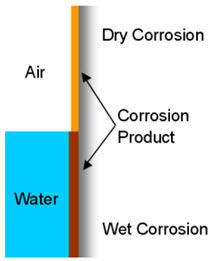
Figure 1: Types of corrosion
Types of Corrosion and Their Mechanisms
Dry corrosion occurs when there is no water or moisture to aid the corrosive process, and the metal oxidizes with the atmosphere alone. In contrast, wet corrosion of metals occurs through electron transfer, involving two primary processes. During oxidation, metal atoms lose electrons at the anode, while the surrounding environment gains these electrons through reduction at the cathode.
Environmental Factors and Atmospheric Conditions
The atmosphere plays a crucial role in corrosion processes. Air contains approximately 21% oxygen, 78% nitrogen, and 1% argon, along with variable amounts of water vapor, ozone, and carbon dioxide. Water is commonly aerated, providing oxygen for reaction with metals.
The corrosiveness of an environment depends on several factors, including oxygen availability, moisture presence, and various pollutants. Corrosion rates are often considered in different atmospheres: industrial, rural, and marine. Each environment contains unique combinations of oxygen and pollutants such as ozone, salt, dust, sulfur dioxide, ammonia, hydrogen, and hydrogen sulfide.
Electrochemical Theory of Wet Corrosion
The fundamental wet corrosion reaction proceeds as: M + H2O (l) → M(OH)2 (s)
This overall reaction occurs through two coupled processes:
- Anode Reaction (oxidation): M → M2+ + 2e‾
- Cathode Reaction (reduction): 2e‾ + H2O + ½O2 → 2OH‾

Figure 2: Wet corrosion
Electrons liberated in the anode reaction flow to the cathode reaction site through the conducting metal. The movement of dissociated ions carries an equal ionic current in the water, with current flow distances varying from microns to many meters.
Additional Reaction Pathways
Several other important reactions occur during wet corrosion processes. These include hydrogen evolution (2e‾ + 2H+ → H2), metal ion reduction (2e‾ + M2+ → M2+), and metal oxide formation (M + H2O → MO + 2H+ + 2e‾).
Equilibrium and Potential Difference
When a metal contacts pure water, ions immediately pass into solution following the reaction M → M2 + + 2e‾. The buildup of negative charge on the metal and metal ions in solution enables a back-reaction, eventually establishing an equilibrium expressed as M ↔ M2+ + 2e‾. At this stage, a steady potential difference exists between metal and solution, depending on the metal type and solution composition.
Corrosion Rate Factors
The rate of wet corrosion often exceeds that of dry corrosion under similar conditions for two primary reasons. First, the dipolar water molecule stabilizes the free metal ions in solution. Second, both the metallic structure and water in contact with it can conduct electric current, facilitating the corrosion process.
立即获取精确的腐蚀性能!
Total Materia Horizon 包含数万种材料在超过 2,000 种介质中的腐蚀行为和性能数据。

申请 Total Materia Horizon免费试用帐户,加入来自全球 120 多个国家超过 500,000 名用户的大家庭。