Steel Deoxidation: Part One
Abstract
This comprehensive examination of steel deoxidation processes explores the fundamental principles and practical applications of oxygen removal from molten metal. The article details various deoxidation methods, focusing on common deoxidizers such as manganese, silicon, and aluminum, while also discussing specialized deoxidants. It analyzes the paradoxical nature of deoxidation processes, equilibrium constants, and their practical implications in different steel grades. Special attention is given to ingot structures and their relationship to deoxidation levels, providing crucial insights for steel manufacturing processes.
Introduction to Deoxidation Processes
Deoxidation, the final stage in steelmaking, involves removing excess oxygen from molten metal through materials with high oxygen affinity. These materials form either gaseous oxides or readily form slags. While Mn, Si, and Al serve as primary deoxidizers, elements like Cr, V, Ti, Zr, and B offer alternative solutions.
Basic Oxygen Furnace Operations
In BOF steelmaking, the steel bath contains 400 to 800 ppm oxygen activity at tapping. Deoxidation occurs during tapping through calculated additions in the tap-ladle. While recarburization may be necessary for below-specification carbon content, large ladle additions are avoided due to temperature considerations.
Ingot Structure Classifications
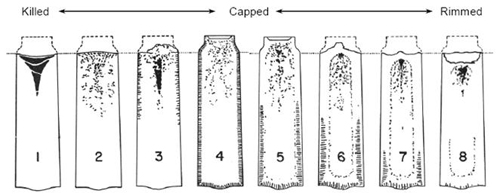
Figure 1: Typical ingot structures from fully killed to violently rimmed
The eight typical ingot conditions demonstrate varying degrees of gas evolution suppression, ranging from dead-killed (No. 1) to violently rimmed (No. 8).
Steel Categories by Deoxidation Level
Rimmed Steel
Minimal deoxidizer additions maintain sufficient oxygen for carbon monoxide evolution. Processing varies with carbon content: 0.12-0.15% for higher ranges, ≤0.10% for lower ranges. The resulting outer ingot skin shows relatively clean metal with low carbon content.
Capped Steel
A rimmed steel variation where initial rimming proceeds normally before termination by cast-iron cap sealing. Preferred for steels exceeding 0.15% carbon, particularly for sheet, strip, wire, and bar production.
Semi-killed Steel
Moderate deoxidation maintains sufficient oxygen for carbon monoxide formation to offset solidification shrinkage. Carbon content typically ranges from 0.15-0.30%, finding wide application in structural shapes.
Killed Steel
Complete deoxidation prevents gas evolution during solidification. Aluminum, combined with manganese and silicon ferro-alloys, serves as the primary deoxidizer. Essential for homogeneous structures in alloy steels, forging steels, and carburizing applications.
Deoxidation Equilibria and Mathematical Models
The fundamental deoxidation reaction follows: MxOy = xM + YO
With equilibrium constant: K = (hM)x(hO)y
Table 1: Complete solubility data for deoxidation products in liquid iron
| Equilibrium constant K* | Composition range | K at 1600°C | log K |
| [aAl]2[aO]4 | < 1 ppm Al | 1.1 x 10-15 | -71600/T + 23.28 |
| [aAl]2[aO]3 | < 1 ppm Al | 4.3 x 10-14 | -62780/T + 20.17 |
| [aB]2[aO]3 | 1.3 x 10-8 | ||
| [aC] [aO]3 | > 0.02% C | 2.0 x 10-3 | -1168/T - 2.07 |
| [aCr]2[aO]3 | > 3% Cr | 1.1 x 10-4 | -40740/T + 17.78 |
| [aMn] [aO] | > 1% Mn | 5.1 x 10-2 | -14450/T + 6.43 |
| [aSi] [aO]2 | > 20 ppm Si | 2.2 x 10-5 | -30410/T + 11.59 |
| [aTi] [aO]2 | < 0.3% Ti | 2.8 x 10-6 | |
| [aTi] [aO] | > 5% Ti | 1.9 x 10-3 | |
| [aV]2[aO]4 | < 0.10 V | 8.9 x 10-8 | -48060/T + 18.61 |
| [aV]2[aO]3 | > 0.3% V | 2.9 x 10-6 | -43200/T + 17.52 |
Activity relationships follow: Hi = fi(wt.% i)
And interaction parameters: (d log fi/d log wt%j) = eji
Table 2: Interaction coefficients for carbon and stainless steels
| Metal | Al | C | Mn | P | S | Si | Ti | H | N | O | Cr | Ni | |
| Carbon steel 1600°C | %i | 0.05 | 0.45 | 0.02 | 0.01 | 0.3 | 0.05 | ||||||
| fi | 1.05 | 1.06 | 1.0 | 1.1 | 1.0 | 1.15 | 0.93 | 1.0 | 0.97 | 0.85 | |||
| ai | 0.053 | 0.45 | 0.022 | 0.01 | 0.345 | 0.046 | |||||||
| Stainless steel 1600°C | %i | 0.05 | 0.45 | 0.02 | 0.01 | 0.3 | 0.05 | 18 | 8 | ||||
| fi | 3.6 | 0.49 | 1.0 | 0.32 | 0.66 | 1.24 | 9.4 | 0.93 | 0.17 | 0.21 | 0.97 | 1.0 | |
| ai | 0.025 | 0.45 | 0.006 | 0.007 | 0.372 | 0.47 | 17.5 | 8.0 |
For practical applications in low alloy steels: KM = (%M)x(%O)y
Example calculation for 0.1% Si at 1600°C: KSi = (%Si)(%O)2 = 2.2 x 10⁻⁵
Therefore, (%O) ≈ 0.015 or 150 ppm
Contemporary Applications and Limitations
While aluminum deoxidation effectively suppresses carbon monoxide formation, certain applications require alternative approaches. Large ingot casting and continuous casting operations often prefer silico-manganese deoxidation or vacuum carbon deoxidation.
立即获取结构钢的精确性能!
Total Materia Horizon 包含 150,000+ 种结构钢的性能数据:化学成分、机械和物理性能、非线性性能等。
申请 Total Materia Horizon免费试用帐户,加入来自全球 120 多个国家超过 500,000 名用户的大家庭。