Epoxy Coated Reinforcement: Advanced Corrosion Protection for Concrete Structures
Abstract
Epoxy coated reinforcement represents a critical advancement in protecting concrete bridge structures from corrosion-related deterioration. This comprehensive analysis examines the corrosion mechanisms affecting steel reinforcement, the development and application of fusion bonded epoxy coated reinforcement (FBECR), and comparative performance evaluations against alternative protection methods. The article explores the physical and electrochemical barrier theories underlying epoxy coating protection, manufacturing processes, and field performance data. Research demonstrates that FBECR significantly reduces corrosion rates in chloride-contaminated environments, though performance varies compared to galvanized and stainless steel alternatives. Understanding these protection mechanisms is essential for optimizing bridge infrastructure durability and minimizing maintenance costs.
The Critical Challenge of Reinforcement Corrosion
The corrosion of steel reinforcement represents the primary and most costly form of deterioration currently impacting reinforced concrete bridge structures. In the United States alone, maintenance and replacement costs for deficient bridges reach billions of dollars annually, highlighting the urgent need for effective corrosion protection strategies.
Reinforced concrete traditionally provides a durable material capable of delivering many years of relatively maintenance-free service life. However, practical experience demonstrates that even high-quality concrete cannot guarantee protection of reinforcing steel from corrosion throughout the extended design life typically specified for modern structures, which ranges from 75 to 120 years, particularly in hostile environments.
Understanding Concrete's Natural Protection System
Concrete creates a naturally protective environment for steel reinforcement through its inherent alkalinity, typically maintaining pH levels between 12.5 and 13. This alkaline environment enables steel to form a passive surface film that effectively prevents corrosion under normal conditions. The structural durability of reinforced concrete depends primarily on two critical factors: concrete quality and adequate cover depth over the reinforcement.
The protective mechanism fails when sufficient chloride levels reach the reinforcement or when atmospheric carbon dioxide carbonates the concrete cover. These aggressive agents destroy the natural protection system, initiating steel corrosion processes. The subsequent expansion of corrosion products damages the surface concrete, creating both structural concerns and costly maintenance requirements that justify implementing additional protection measures for structures in aggressive environments.
Historical Development of Epoxy Coating Technology
During the 1970s and 1980s, the construction industry widely accepted that epoxy coatings would provide superior corrosion protection for reinforcing steel embedded in concrete. This belief led to routine implementation of epoxy coated reinforcement in North American bridge decks and substructures. Concurrently, cathodic protection systems were also deployed to safeguard bridge substructures from corrosion-related deterioration.
The pursuit of innovative solutions to eliminate or significantly reduce reinforced concrete structure deterioration drove the development of advanced protection technologies. While increasing concrete cover depth offers enhanced protection, this approach increases both dead load and construction costs without providing structural benefits. Epoxy coatings emerged as an attractive alternative, offering corrosion protection with minimal impact on construction costs.
However, field experience revealed potential limitations of epoxy coating systems. Breaks in the epoxy coating at cracked locations, combined with high chloride concentrations, can result in localized steel reinforcement corrosion that affects overall bridge performance. Additionally, epoxy coatings in aging bridge decks may become brittle and eventually delaminate from the steel reinforcement, compromising their protective effectiveness.
Fundamental Corrosion Mechanisms and Protection Principles
The epoxy coating functions as a physical barrier designed to protect reinforcing steel from aggressive environmental conditions. Under normal circumstances, concrete maintains a passive, electrically neutral film around embedded steel due to the material's high pH environment. However, when chlorides penetrate the concrete in the presence of water and oxygen, the pH decreases and the protective passive film deteriorates, initiating corrosion reactions at the steel surface.
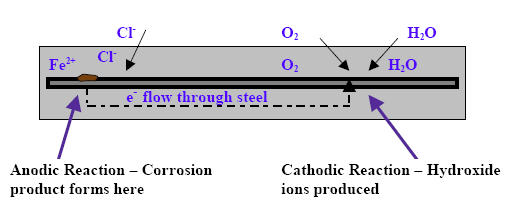
Figure 1: Corrosion reaction
The corrosion process involves simultaneous cathodic and anodic reactions, with the steel functioning as an electrode coupling these complementary processes. In the anodic reaction, iron atoms lose electrons through oxidation after passive film destruction, following the reaction:
Fe → Fe²⁺ + 2e⁻
The resulting iron ions react with hydroxides to produce ferrous hydroxide:
Fe²⁺ + 2OH⁻ → Fe(OH)₂
Subsequently, ferrous hydroxide reacts with diffused oxygen, producing rust as the final corrosion product:
4Fe(OH)₂ + O₂ → 2Fe₂O₃(rust) + 4H₂O
The cathodic reaction involves electron flow through the steel, where electrons react with water and oxygen to form hydroxide ions:
4e⁻ + 2H₂O + O₂ → 4OH⁻
This electrochemical process creates a complete electric circuit through hydroxide ion conduction via the electrolyte (pore water in concrete), establishing what engineers term a corrosion cell.
Theoretical Foundation of Epoxy Protection Systems
Two complementary theories explain the protection capability of epoxy coated reinforcement systems. The Physical Barrier Theory describes how epoxy coating acts as a barrier preventing chloride ions and other aggressive substances from contacting the steel surface. The Electrochemical Barrier Theory explains how epoxy coating functions as a high-resistance barrier, reducing corrosion by increasing electrical resistance between neighboring coated steel locations where cathodic reactions occur.
These theoretical frameworks provide sound scientific reasoning for implementing epoxy coated reinforcement as a protective measure for structures exposed to marine environments and other aggressive conditions.
Fusion Bonded Epoxy Coated Reinforcement Development
Fusion bonded epoxy coated reinforcement (FBECR) emerged in the late 1960s and early 1970s as a direct response to severe rebar corrosion problems in bridge decks. This technology has evolved into a well-established durability solution for protecting reinforced concrete structures in high chloride environments.
Early research programs evaluated various coating materials and concluded that fusion bonded epoxy powder coatings provided the optimal combination of properties for protecting rebar in chloride-contaminated concrete. Subsequent laboratory and field studies consistently demonstrated that FBECR performs exceptionally well compared to both unprotected steel reinforcement and galvanized alternatives.

Figure 2: Method of manufacture of fusion bonded epoxy resin coated reinforcing bar
The FBECR manufacturing process involves applying specially formulated epoxy powder resins to blast-cleaned and heated steel reinforcement. The residual heat in the reinforcement polymerizes the epoxy, creating a robust coating that conforms to the reinforcement profile. FBECR is often subsequently cut and bent to meet specific structural element requirements. Non-flexible FBE coatings are also available for applications requiring improved bonding performance, where reinforcement is either pre-formed before coating or utilized in straight lengths.
Protection Mechanisms of FBECR Systems
Corrosion of uncoated reinforcement typically results from localized loss of the passive surface film when chloride ions penetrate through cover concrete, or when local concrete alkalinity decreases due to carbonation. The exposed steel subsequently corrodes as an anode driven by the relatively large cathode of surrounding passive steel, resulting in pitting corrosion known as macrocell corrosion.
Epoxy coating on steel creates an exceptionally effective barrier against aggressive agents, particularly chloride ions, which cannot easily diffuse through continuous epoxy coatings. This barrier effect directly protects the underlying steel surface from corrosive attack.
The system design accounts for normal coating damage during assembly and concrete casting operations. While steel can corrode at coating discontinuities, the extent of corrosion remains controlled by surrounding intact epoxy layers that restrict cathodic reactions and prevent macrocell corrosion development. The steel surface at coating holes must sustain both anodic and cathodic reactions, and because cathodic reactions are significantly less efficient than anodic reactions, the corrosion rate near coating defects is 10 to 100 times lower than rates observed in uncoated reinforcement.
Performance Evaluation and Comparative Analysis
Extensive research programs have evaluated FBECR performance, consistently concluding that it performs effectively and reduces deterioration rates in reinforced concrete containing high chloride levels. Comparative analysis of laboratory research and natural weathering behavior of FBECR structures demonstrates that thousands of FBECR installations perform according to laboratory-based study predictions.
Comprehensive research projects have assessed the comparative performance of carbon steels, FBECR, galvanized rebar, and stainless steel rebar systems. Results consistently show that in chloride-contaminated concrete environments, FBECR outperforms both carbon steel and galvanized steel alternatives, though it demonstrates inferior performance compared to austenitic stainless steel systems.
Table 1. Comparison of cost and performance of different reinforcing bar materials
| Type of rebar | Relative cost | Corrosion protection method | Performance in carbonated concrete | Performance in concrete with high Cl | Other Factors |
| Carbon Steel | 1.0 | Cover concrete provides protection | Poor(Depends on concrete) | Poor(Depends on concrete) | Additional protection needed |
| Galvanized | 1.5 | Initially a barrier and subsequently sacrificial cathodic protection | Good | Poor | Reduced rebar ductility |
| FBECR | 2.0 | Barrier coating | Good | Good | Reduced bond to concrete |
| Austenitic Stainless Steel | 8.1 | Passive | Excellent | Excellent | Electrical Isolation form other rebar is required |
The performance comparison reveals significant variations in both cost and effectiveness among different reinforcement protection strategies. Carbon steel, serving as the baseline with relative cost of 1.0, relies entirely on cover concrete for protection and demonstrates poor performance in both carbonated concrete and high-chloride environments. Galvanized reinforcement, at 1.5 times the cost of carbon steel, initially provides barrier protection followed by sacrificial cathodic protection, showing good performance in carbonated concrete but poor performance in high-chloride conditions while reducing rebar ductility.
FBECR, costing approximately twice as much as carbon steel, provides effective barrier coating protection with good performance in both carbonated concrete and high-chloride environments, though it may reduce bond strength to concrete. Austenitic stainless steel, despite its significantly higher cost at 8.1 times that of carbon steel, offers excellent performance in all conditions through passive corrosion resistance, though it requires electrical isolation from other rebar types.
Conclusion and Future Considerations
Epoxy coated reinforcement technology represents a significant advancement in protecting concrete infrastructure from corrosion-related deterioration. The combination of physical and electrochemical barrier protection mechanisms provides effective corrosion control in aggressive environments, particularly those with high chloride concentrations. While FBECR systems demonstrate superior performance compared to conventional carbon steel and galvanized alternatives, careful consideration of application requirements, environmental conditions, and long-term performance expectations remains essential for optimal protection system selection.
Understanding the fundamental corrosion mechanisms, protection theories, and comparative performance characteristics enables engineers to make informed decisions regarding reinforcement protection strategies, ultimately contributing to enhanced infrastructure durability and reduced lifecycle maintenance costs.
立即获取精确的腐蚀性能!
Total Materia Horizon 包含数万种材料在超过 2,000 种介质中的腐蚀行为和性能数据。
申请 Total Materia Horizon免费试用帐户,加入来自全球 120 多个国家超过 500,000 名用户的大家庭。