Corrosion Behaviour of Copper Alloys: Part One
Abstract
Copper demonstrates exceptional corrosion resistance in marine environments while exhibiting strong resistance to various localized corrosion forms that could potentially cause failure. When exposed to atmospheric conditions, copper undergoes distinctive visual transformations, developing a brown patina within days that gradually evolves into the characteristic green color over 7-9 years. The corrosion mechanism of copper is strongly dependent on chloride ion presence, with three reversible electro-dissolution reactions governing the process. Copper alloys maintain their corrosion resistance through protective surface film formation and demonstrate significantly lower long-term corrosion rates compared to short-term exposure data. Long-term studies reveal steady-state corrosion rates of approximately 1 mpy for most copper alloys and 0.05 mpy for copper-nickel alloys in seawater applications.
Understanding Copper Corrosion Mechanisms
Corrosion represents the electrolytic action of moisture and dissolved atmospheric ions on metals. Outdoor environments present unique challenges through salty coastal atmospheres, acid rain, and ice exposure, leading to material degradation and eventual failure. The corrosion mechanism of copper demonstrates strong dependence on chloride ion presence, with several literature models describing copper corrosion in chloride-containing media.
The primary differences among available models center on descriptions of initial electro-dissolution reactions of bare copper. Three reversible mechanisms have been identified: reactions showing direct cupric chloride formation from copper, and a third reaction representing copper dissolution to cuprous chloride as the initial step.
Cu + 2Cl- ↔ CuCl2- + e-
Cu ↔ Cu+ + e-
Cu++ 2Cl- ↔ CuCl2-
Cu + Cl- ↔ CuCl + e-
CuCl + Cl- ↔ CuCl2-
Marine Environment Performance of Copper Alloys
The exceptional marine corrosion resistance of copper has been recognized and utilized effectively for centuries. Copper alloys used in seawater service demonstrate low general corrosion rates while maintaining high resistance to numerous localized corrosion forms that can cause rapid failure.
Copper-based alloys find frequent application in seawater systems including heat exchangers, pumps, valves, pipes, and fasteners. Depending on application requirements and mechanical characteristics needed, CuNi alloys (such as CuNi10Fe1Mn) or Nickel Aluminium Bronzes are commonly selected. Despite recent emergence of materials offering improved properties including stainless steels, nickel-based alloys, or titanium, copper alloys continue widespread use due to attractive pricing and interesting mechanical characteristics combined with relatively good seawater corrosion resistance.
Protective Surface Film Formation
Copper alloys achieve corrosion resistance through protective surface film formation. These films are visible to the naked eye, form gradually over time in both atmospheric and seawater environments, and result from reactions between air or seawater and the alloy surface.
In atmospheric conditions, copper develops a brown patina within days, gradually weathering into a green patina. Continued weathering converts sulfide films to basic copper sulfate patina, which when complete, produces the distinctive light green color of aged copper roofs. In marine climates, surface patina also contains copper chloride. The eventual light green patina development requires 7 to 9 years in marine environments.
Long-term Corrosion Performance Data
Exposure test data spanning up to 20 years duration at various marine sites demonstrate high marine atmospheric corrosion resistance for diverse copper alloys, with corrosion rates ranging from 1.3-26x10⁻⁴ mm/year, except for alloys susceptible to dezincification where corrosion rates were significantly higher.
Reinhart's research revealed that copper and its alloys containing aluminum, silicon, tin, beryllium, and nickel exhibited significantly lower long-term corrosion rates after 18 months compared to specimens measured after only 6 months of seawater exposure.
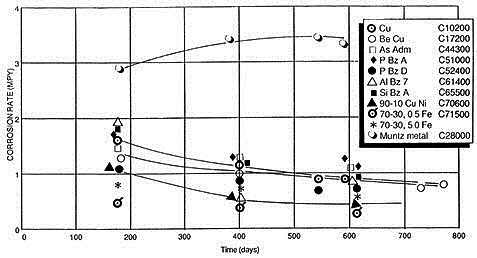
Figure 1: Corrosion rates for copper alloys during up to 800-day seawater exposures
The sole exception was Alloy C28000 (Muntz metal), which demonstrated slightly higher corrosion rates after 18 months compared to 6 months. Alloy C44300 (Admiralty) belonged to the group characterized by decreasing corrosion rates over time. Long-term, steady-state corrosion rates for copper and copper alloys, excluding Muntz metal, approximate 1 mpy (0.025 mm/y) or less, while copper-nickel alloys achieve approximately 0.05 mpy (0.001 mm/y) within velocities each alloy can tolerate without protective corrosion product film damage.
Importance of Long-term Testing
Short-term corrosion test data (less than 1 year) on copper alloys in seawater, while useful for specific alloy-to-alloy research comparisons, can be misleading when used for service life estimation. Short-term data tend to underestimate long-term durability significantly. Only long-term, steady-state corrosion rates can provide reasonable service life projections for copper alloys.
Acesse Propriedades Precisas de Corrosão Agora!
Total Materia Horizon contém informações sobre comportamento de corrosão e propriedades para centenas de milhares de materiais, em mais de 2.000 meios.

Obtenha uma conta de teste GRATUITA na Total Materia Horizon e junte-se a uma comunidade de mais de 500.000 usuários de mais de 120 países.