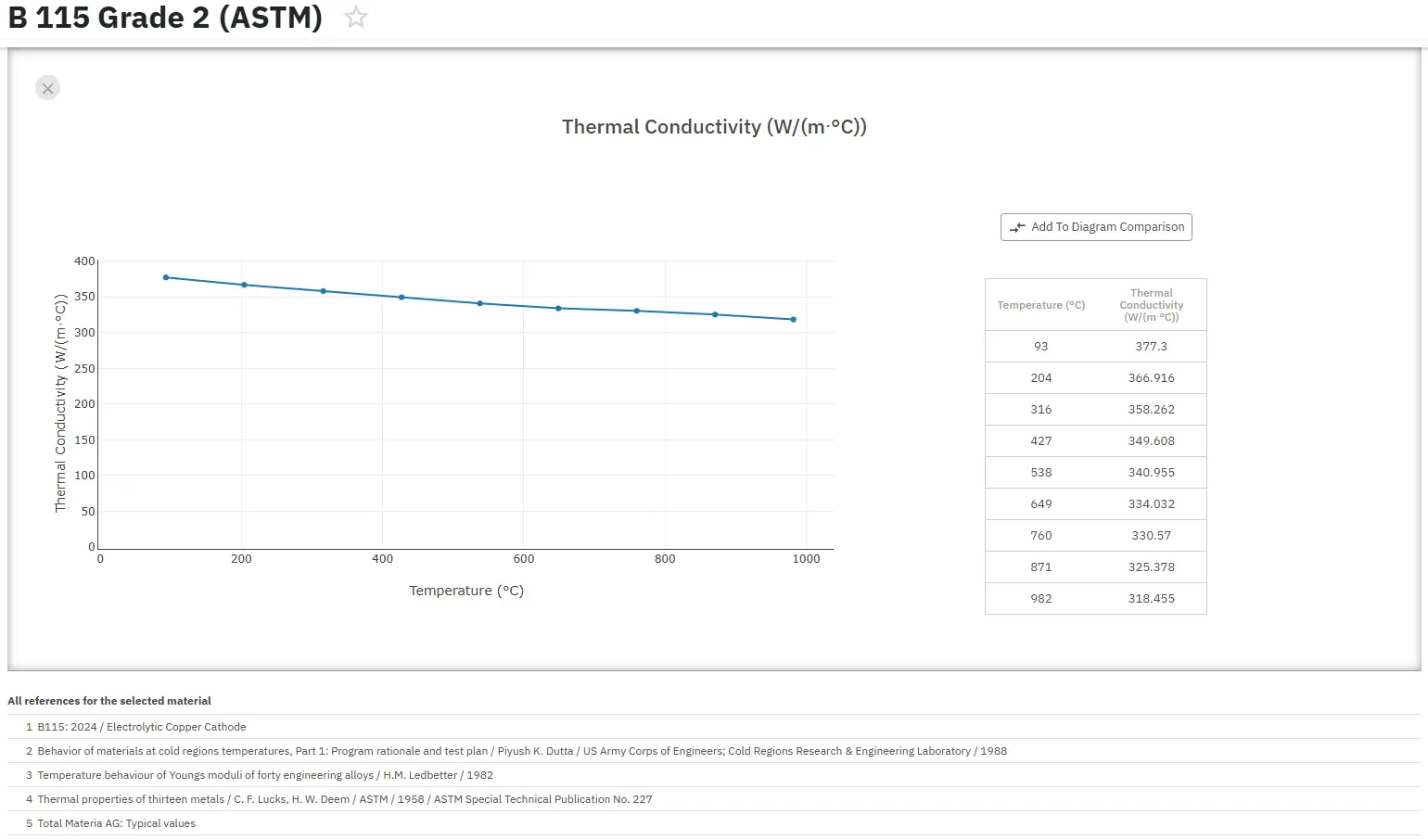
Copper Alloys Applications in Electrical Engineering
Abstract
Copper alloys represent critical materials in electrical engineering applications where pure copper's mechanical properties are insufficient for demanding operating conditions. While pure copper offers the highest electrical conductivity among commercial metals, many electrical applications require enhanced mechanical properties and elevated temperature capabilities while maintaining acceptable conductivity levels. This comprehensive review examines high copper alloys, including solid solution hardened and precipitation hardened variants, which achieve mechanical properties equal to or exceeding other engineering metals while retaining conductivities suitable for electrical applications. The article explores strengthening mechanisms, relaxation resistance characteristics, and specific applications ranging from connector pins to heavy electrical switchgear, demonstrating how modern copper alloy technology meets the evolving demands of electrical engineering.
Introduction to High Copper Alloys in Electrical Applications
Pure copper possesses the highest electrical conductivity of any commercial metal, making it the preferred material for power and telecommunications cables, magnet winding wire, printed circuit board conductors, and numerous other electrical applications. Copper demonstrates sufficient strength, ductility, and hardness for standard applications at operating temperatures up to 100°C. However, many advanced electrical applications require copper to exhibit higher mechanical properties and capability for use at elevated operating temperatures while retaining the excellent conductivity for which it is initially selected.
The copper industry has invested decades of research effort to create materials capable of meeting these demanding requirements. The products of this extensive research are found in the large variety of high copper alloys—materials whose properties equal or, in some cases, exceed those of many other engineering metals, yet maintain conductivities high enough for electrical applications.
In terms of composition for wrought product forms such as rod, bar, sheet, and strip, these high copper alloys are defined as having designated copper contents less than 99.3% but more than 96% and do not fall into any other copper alloy classification. Cast high copper alloys (C81400-C83299) have designated copper contents exceeding 94%, to which silver may be added for special properties. Their relatively high copper content gives this family of copper alloys their superior conductivity characteristics.
Strengthening Mechanisms in Copper Alloys for Electrical Applications
Pure copper serves as the optimal material for electric current conductors, combining high electrical conductivity with reasonable cost. However, many wire and cable applications require strength levels that exceed those attainable with pure copper wire, particularly in applications such as connector pins. In these cases, copper alloys become necessary to meet performance requirements.
Strength increases in copper alloys are achieved through two different metallurgical effects: solid solution hardening and precipitation hardening. Brass and bronze represent widely used solid solution hardened alloys, while certain high copper alloys with low contents of alloying elements such as nickel, silicon, and chromium are precipitation hardened and offer an attractive combination of high strength, good electrical conductivity, and relaxation resistance.
The high copper alloy family includes, in wrought forms, cadmium coppers (C16200 and C16500), beryllium coppers (C17000-C17500), chromium coppers (C18100-C18400), zirconium copper (C15000), chromium-zirconium copper (C14500), and combinations of these and other elements. Alloy C18000, another member of the group, contains nickel, silicon, and chromium. Fewer cast high copper alloys exist, although the beryllium copper family is well represented in casting applications.
Solid Solution Hardened Copper Alloys
Fundamental Hardening Mechanisms
The copper lattice can dissolve certain amounts of atoms from other metals, such as tin, zinc, and magnesium. These atoms occupy the lattice sites of copper atoms, creating what is called a solid solution. The copper lattice in the vicinity of these foreign atoms becomes distorted through expansion when the atoms are larger than copper atoms, such as zinc and magnesium. When the atoms are smaller than copper atoms, such as tin, nickel, and aluminum, the lattice distortion manifests as contraction.
In both cases, the material's resistance to deformation increases compared to pure copper, effectively making the material harder. This type of alloy is termed "solid solution hardened alloys." Some elements can be dissolved in copper in high percentages. According to equilibrium phase diagrams, the maximum solubility of zinc is 39.0% and tin is 15.8%. Standard brasses with 30 or 36% zinc and bronzes with 5, 6, and 8% tin are frequently applied in the electronics industry when pure copper's strength is insufficient.
Strength Development and Conductivity Trade-offs
To achieve high-strength wire, in addition to the solid solution hardening effect, a high degree of cold drawing deformation is necessary. Thin wires of phosphor bronze easily achieve strength values of 1000 MPa due to cold drawing processes. However, lattice distortion caused by alloyed elements decreases electrical conductivity. The primary disadvantage of solid solution hardened alloys is their low electrical conductivity, approximately 25% IACS for brass CuZn36 and about 14% IACS for phosphor bronze CuSn6. This conductivity decrease results from lattice distortion caused by alloying atoms.
To minimize the drop in electrical conductivity while increasing strength, solid solution hardened high copper alloys with low content of alloyed elements are applied. Examples include CuSn0.15, CuSn0.3, and CuMg0.1. A weakness of all solid solution hardened alloys is their insufficient relaxation resistance at slightly elevated service temperatures, beginning at approximately 60°C. To overcome this limitation, precipitation hardened alloys become necessary.
Precipitation Hardened High Copper Alloys
Precipitation Hardening Mechanisms
The ability to dissolve other types of atoms generally increases at elevated temperatures. When temperature decreases, the solubility limit is exceeded, enabling the utilization of this phenomenon to generate precipitations through an annealing procedure at temperatures below the solubility limit. The atoms form precipitations, creating a second phase or intermetallic compound. The size of these particles is typically less than 100 nanometers.
As the atoms leave the lattice, the lattice distortion is eliminated, and the electrical conductivity of the material increases. Cold deformation after solution annealing but prior to age annealing supports the formation of small-sized and homogeneously distributed precipitations. The precipitates increase the base strength of the material and influence the strengthening behavior, hardening the material. For this reason, such alloys are called "precipitation hardened alloys."
Advantages in High-Temperature Applications
A significant advantage of precipitation hardened alloys is their relaxation resistance. When the material is exposed to elevated service temperatures, the precipitates do not dissolve, and the increased base hardness is maintained. Wires of two alloys, CuFe2P and CuNi3SiMg, are already being used for connector pins. Since the content of alloyed elements in these alloys is low, they are classified as high copper alloys and are specifically called "precipitation hardened high copper alloys."
Relaxation Resistance in High-Performance Applications
Demanding service conditions require high-performance materials, particularly when the automotive industry designs connectors in engine compartment environments. These conditions include impacts requiring high strength and ductility, vibrations requiring high fatigue strength, and elevated temperatures requiring relaxation resistance.
The strength of solid solution hardened alloys results from lattice deformation during the cold drawing process. During exposure to elevated temperatures, the deformation thermally recovers (relaxes), and the strength decreases toward the material's base strength value. Since this base strength is low, solid solution hardened alloys suffer from severe relaxation. While precipitation hardened alloys are subject to the same degradation mechanism, their base strength is much higher, resulting in good, and in some cases, very good relaxation resistance.
Applications of High Copper Alloys in Electrical Engineering
Terminals and Connectors
Several typical applications encompass a wide variety of designs and demands to meet product requirements. Many applications listed are ordinarily satisfied by electrical coppers (UNS C10100-C12000), with high copper alloys used only when enhanced properties are needed and somewhat lower electrical or thermal conductivity can be tolerated.
Terminals and connectors for electrical, electronic, and automotive applications represent the bulk of high copper alloy usage. Most of these are manufactured from brass or, for more demanding applications, phosphor bronze. High copper alloys such as beryllium copper, copper-nickel, and others are reserved for severe duty applications, especially regarding stress relaxation resistance. Factors such as formability, strength, and conductivity play crucial roles in materials selection decisions, with designers typically working with alloy suppliers for detailed property requirements.
Specialized Electrical Components
Springs for relay contacts and switchgear also utilize less-costly alloys for commodity-type products, with high copper alloys employed when specific needs arise. Integrated circuit lead frames are manufactured from specialty alloys designed for both connector-related properties and compatibility with packaging requirements.
Busbars, unless welded, are typically manufactured from electrolytic tough pitch copper (C11000) or, for maximum conductivity, electronic or oxygen-free high conductivity coppers. Where mechanical requirements demand higher strength, dilute alloys such as silver-bearing copper or high copper alloys can be considered. Welded or brazed busbars require either oxygen-free copper or deoxidized copper.
Industrial and Automotive Applications
Rotor bars are normally manufactured from pure copper unless strength requirements dictate higher mechanical properties. Armatures are also made from pure copper unless higher strength or annealing resistance is needed, in which case silver-bearing copper can be considered. Commutators utilize silver-bearing copper (C11400) for its annealing resistance.
Spot welding electrodes and seam welding wheels employ various grades listed by the Resistance Welding Manufacturers Association (RWMA), including chromium coppers (Class II) and dispersion-strengthened coppers (Class III), among several others. Heavy electrical switchgear can specify chromium copper, zirconium copper, beryllium coppers, and other high copper alloys, depending on strength requirements.
Strip Metals for Interconnection Applications
The use of strip metals for connector springs, terminals, contacts, and switches is well established in the interconnection industry. This is particularly true for copper alloys because of their desirable combination of conductivity, strength, and formability. The connector designer approaches each new assignment with information on the connector's desired mechanical characteristics, operating environment, and life expectancy.
The designer's task is to find a specific design that can be reliably and economically manufactured. To accomplish this goal, they must identify the copper alloy and temper that will allow the connector to be manufactured, assembled, and used successfully throughout its intended life, all at minimum cost.
Advanced High-Performance Copper Alloy Developments
A new generation of high-performance copper alloy wire is attracting attention from the electronics industry. Excellent material properties have been obtained through precipitation hardening effects. These alloys are recognized in the electronics industry as precipitation hardened copper alloy strip for connectors and electrical contacts. These alloys are now available as wire, which is often further processed into square-shaped connector pins.
Wire manufactured from precipitation hardened high copper alloys such as UNS C70250 attracts increasing attention from connector designers. It has been found suitable for application as square-shaped connector pins, offering numerous attractive properties including high strength up to 900 MPa, reasonable electrical conductivity (45 to 55% IACS), high relaxation resistance, excellent bending properties, sufficient ductility for further processing, and ease of galvanizing.
Conclusion
High copper alloys represent essential materials for modern electrical engineering applications where pure copper's mechanical properties are insufficient. Through solid solution hardening and precipitation hardening mechanisms, these alloys achieve the optimal balance between electrical conductivity and mechanical performance required for demanding applications. The continued development of precipitation hardened high copper alloys demonstrates the industry's commitment to meeting evolving performance requirements while maintaining the fundamental electrical properties that make copper alloys indispensable in electrical engineering applications.
Acesse Propriedades Precisas de Ligas de Cobre Agora!
Total Materia Horizon contém informações de propriedades para mais de 30.000 ligas de cobre: composição, propriedades mecânicas, físicas e elétricas, propriedades não lineares e muito mais.
Obtenha uma conta de teste GRATUITA na Total Materia Horizon e junte-se a uma comunidade de mais de 500.000 usuários de mais de 120 países.