Boron in Steel: Part One
Abstract
Boron is a critical alloying element that significantly enhances steel hardenability when added in optimal concentrations of 0.0003 to 0.0030% B. At atmospheric pressure and 0°C, boron exists as a solid material that can be supplied to steelmakers as ferroboron or proprietary alloys. The element's effectiveness stems from its ability to segregate at austenite grain boundaries, retarding gamma-alpha transformations and preventing ferritic reactions. This comprehensive analysis examines boron's fundamental properties, solubility behavior in iron, available commercial forms, and optimal application methods for maximizing steel performance while maintaining cost-effectiveness in industrial steelmaking operations.
Introduction to Boron Steel Applications
The strategic addition of boron to steel represents one of the most cost-effective methods for improving hardenability in modern metallurgy. The name "boron" derives from the Armenian-Persian linguistic tradition, specifically from "buraq" or "burah," referring to borax (sodium tetraborate decahydrate), one of the most recognized boron compounds in history. During the Middle Ages, borax was exported under the commercial name "Tincal" from Central Asian salt lakes to European markets, where metalworkers utilized it as a soldering and melting aid.
Fundamental Properties of Boron for Steel Applications
Natural Occurrence and Key Boron Minerals
Boron comprises approximately 10 parts per million of Earth's outer crust and never occurs naturally in its elementary state, always appearing combined with oxygen. The most technically significant boron-containing minerals include:
- Borax (Tincal): Na₂O × 2B₂O₃ × 10H₂O
- Tincalconite: Na₂O × 2B₂O₃ × 5H₂O
- Kernite (Rasorite): Na₂O × 2B₂O₃ × 4H₂O
- Boracite: 5MgO × MgCl₂ × 7B₂O₃
- Colemanite: 2CaO × 3B₂O₃ × 5H₂O
- Sassolin: B₂O₃ × 3H₂O
The world's largest boron deposits are strategically located in Kazakhstan, California, Argentina, and Turkey. H. Davy first successfully produced boron in 1808 in its amorphous form, establishing the foundation for modern boron metallurgy.
Physical and Chemical Characteristics
Boron stands as the only nonmetal element in the third main group of the periodic table. Under atmospheric pressure at 0°C, boron maintains its solid state. The element possesses six isotopes, several of which exhibit radioactive properties with extremely short half-lives of less than one second.
Table 1. Boron Structural Data
The structural variations of boron demonstrate distinct characteristics:
| Modification | Amorphous | Crystalline |
| Color | Brown | Black-grey |
| Phase | α, β | γ |
| Temperature Range (°C) | 800-1100, ≥1300 | 1100-1300 |
| Lattice Type | Rhombohedral | Tetragonal |
| Dimensions | a=1.789 nm, b=0.895 nm, c=1.015 nm | - |
Table 2. Boron Mechanical Properties
The mechanical properties vary significantly between amorphous and crystalline forms:
| Property | Amorphous | Crystalline |
| Density (g/cm³) | 1.73 | α: 2.46, β: 2.35, γ: 2.37 |
| Hardness | 9.3 | - |
| Tensile Strength (MPa) | 1.6-2.4 (amorphous), 2.6-3.1 (fibers) | - |
| Compressive Strength (MPa) | 0.5 | - |
| Elasticity Modulus (MPa) | 440 | - |
Critical thermodynamic characteristics include:
- Flame Point: 70°C
- Melting Point: 2300°C
- Boiling Point: 2550°C
- Thermal Expansion Coefficient: 1.1-8.3 nm/m·K (20-750°C range)
- Latent Heat of Vaporization: 34,900 kJ/kg
- Latent Heat of Fusion: 22,000 kJ/kg
- Latent Heat of Combustion: 5.4 kJ/kg
- Diffusion Coefficient in γ-Fe: D = 0.002 e^(-21000/RT)
Boron Diffusion and Steel Hardenability Mechanisms
According to research by P.E. Brushby and colleagues, boron exhibits diffusion velocities comparable to carbon, with a diffusion coefficient of D = 0.002e^(-21000/RT). This similarity proves crucial for steel hardenability enhancement. With carbon contents up to 0.43%, boron solubility in the austenite lattice remains independent of carbon concentration. Conversely, carbon diffusion remains unaffected by boron contents up to 0.009%, demonstrating excellent compatibility between these elements.
Boron Solubility Behavior in Pure Iron Systems
Atomic Position Theories in Iron Crystal Lattice
The scientific community maintains differing perspectives regarding boron atom positioning within iron crystal lattices. Jandeska and I.J. Morral concluded through diffusion coefficient comparisons that boron atoms dissolve interstitially within the γ-Fe lattice. C.C. McBride, J.W. Spretnak, and R. Speiser reached identical conclusions by comparing theoretical boron atomic radii with interatomic distances in γ-Fe lattices, though these geometric analyses overlook physical and chemical bonding mechanisms.
R.M. Goldhoff and J.W. Spretnak employed X-ray deflection measurements, discovering that gamma iron lattice parameters decrease in boron's presence. They interpreted this as evidence supporting substitutional dissolution of boron atoms in austenite lattices. Given the atomic radii of boron and iron, they concluded that lattice locations prove more favorable than intermediate positions for boron atoms.
Their research revealed that lattice parameter differences between pure iron and boron-containing iron decrease with rising temperatures. This observation led them to conclude that increasing temperatures cause more boron atoms to migrate from lattice positions to grain boundaries, where boron segregations have been experimentally confirmed.
Fe-B Binary System Analysis
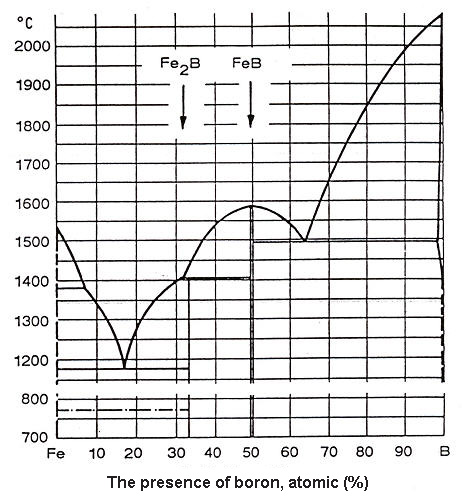
Figure 1: Kubaschewsky Fe-B Phase Diagram
O. Kubaschewsky's Fe-B binary system diagram reveals two distinct eutectics: one at 17 atomic percent boron and another at 63.5% boron. Between these eutectics, liquidus temperatures vary from 1149°C (eutectic temperature at 17 atomic percent boron) to 1590°C for alloys containing 50 atomic percent boron.
Iron's melting temperature can be reduced by 150°C to a maximum of 350°C (at 17 atomic percent boron) through the addition of 5 to 30 atomic percent boron. Beyond the second eutectic, increasing boron proportions in ferroboron cause liquidus temperatures to rise steadily and almost linearly toward pure boron's melting temperature.
In this system, iron boride (FeB) contains 16.23 weight percent boron, exhibits rhombic lattice structure, and demonstrates extreme hardness of 2300 HV0.2. Iron-II-boride (Fe₂B) contains 8.83 weight percent boron, displays tetragonal lattice structure, and maintains hardness between 1800 and 2000 HV0.2, making it preferable when avoiding FeB formation.
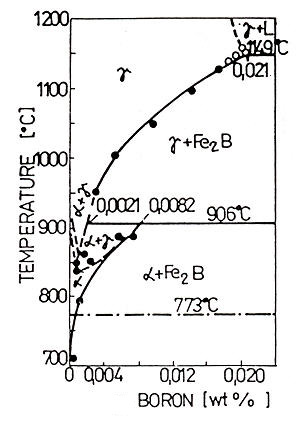
Figure 2: Equilibrium Phase Fe-B Diagram for Low Boron Concentrations
Houndremon's binary phase diagram for the iron-rich corner demonstrates that maximum boron solubility reaches 0.021% at 1149°C in low concentration regions. As temperatures decrease, solubility correspondingly decreases to 0.0021% at 906°C. This temperature represents a peritectoid reaction point at 0.0082% B, where boron solubility in γ-Fe suddenly decreases, leaving only approximately 0.0004% boron substitutionally dissolved at 710°C.
Optimal Boron Content for Maximum Steel Hardenability
J.W. Spretnak investigated boron's potential to form films surrounding austenitic grains in steel, concluding through geometrical considerations that such film formation proves impossible. Instead, researchers discovered point Fe₂B precipitation at grain boundaries. In steel systems, boron disperses within the matrix as Fe₂B boride particles measuring 20 to 30×10⁻⁸ cm, along with free boron that predominantly segregates around primary austenite grain boundaries.
This strategic arrangement of small amounts of soluble boron along grain boundaries effectively retards γ-α transformations through diffusion mechanisms, preventing ferritic reactions and thereby enhancing steel hardenability. Experience-based research indicates that optimal boron quantities for achieving maximum hardenability range from 0.0003 to 0.0030% B.
Boron additions exceeding these established values deteriorate hardenability because excess boron atoms precipitate as surface-centered cubic Fe₂₃(CB)₆ borocarbide, which can serve as preferential nucleation sites for ferrite formation.
Commercial Boron Addition Agents for Steel Production
Ferroboron: The Standard Addition Agent
Boron reaches steelmakers primarily as ferroboron or through various proprietary alloys. Selection depends on steelmaking practices, product mix requirements, production volumes, individual operator experience and preferences, and economic considerations. Steelmakers should select addition agents that provide the highest and most reliable recovery rates consistent with overall melt shop economics.
Ferroboron represents the lowest-cost addition agent available. Standard grades contain relatively high boron content, with incremental levels between 12 and 24% B. Major impurities include carbon (0.10-1.5%), silicon (0.30-4.0%), and aluminum (0.5-8.0%). A typical analysis includes 18.0% B, 0.50% C, 0.50% Si, 0.2% Al, 0.03% P, and 0.01% S, with all values except boron representing maximum concentrations.
Ferroboron is supplied in lump form (2-inch or 1-inch × down sizing), packaged in 250 kg or 500 lb steel drums, or supersacks (bulk bags) with capacities up to 3000 lb (1360 kg). Many customers specify minimum size limits, such as 5 mm (0.2 inch), to minimize fine material quantities that can produce poor recoveries in less controlled melting practices. Cored wire forms of ferroboron are also commercially available.
Proprietary Boron Addition Agents
Since ferroboron lacks appreciable concentrations of protective elements, it requires greater care than proprietary alloys to achieve adequate and consistent results. Ferroboron is typically added after other oxygen/nitrogen scavengers, such as ferrotitanium.
Proprietary boron addition agents command higher initial costs than ferroboron but are often preferred for their superior efficiency, application ease, and more consistent results. All proprietary agents contain varying proportions of oxygen and/or nitrogen scavengers including titanium, aluminum, silicon, and zirconium. These elements generally demonstrate even greater affinity for oxygen and nitrogen than boron itself.
The most common proprietary addition agent typically contains 2.0% B, 15% Al, 30% Ti, 10% Si, with the balance being iron. This product's high scavenger-to-boron ratio ensures effectiveness across all boron steel applications, provided adequate prior deoxidation has been achieved.
Various other proprietary boron addition alloys are available with boron contents ranging between 0.5% and 4%. Generally, higher ratios of boron to scavenger elements require greater care to ensure adequate boron recovery in the final steel product.
Proprietary boron addition agents are marketed in lump form (1-1/4 inch and 2 inch × down), packaged in bags, cans, or large drums for convenient handling and application.
Conclusion
Boron's role in steel hardenability enhancement represents a critical aspect of modern metallurgical practice. Through careful control of addition quantities within the optimal range of 0.0003 to 0.0030% B, steelmakers can achieve significant improvements in hardenability while maintaining cost-effectiveness. The choice between ferroboron and proprietary addition agents depends on specific operational requirements, with each offering distinct advantages in terms of cost, reliability, and ease of application. Understanding boron's fundamental properties, solubility behavior, and optimal application methods enables steelmakers to maximize the benefits of this powerful alloying element in their production processes.
Leia mais
Encontre Composições Precisas de Materiais Instantaneamente!
Total Materia Horizon contém composições químicas de centenas de milhares de materiais e substâncias, bem como suas propriedades mecânicas e físicas e muito mais.
Obtenha uma conta de teste GRATUITA na Total Materia Horizon e junte-se a uma comunidade de mais de 500.000 usuários de mais de 120 países.