Liquid Salt Bath Nitriding
Abstract
Liquid salt bath nitriding, also known as ferritic nitrocarburizing (FNC), is a widely adopted case hardening technique that enhances material properties through thermochemical processes. This treatment provides increased surface hardness, improved wear resistance, and enhanced corrosion protection while preserving core ductility. The process involves diffusing nitrogen and carbon into the material surface at relatively low temperatures, forming a thin outer layer of epsilon iron nitride (Fe3N). This comprehensive overview examines the fundamental principles, process advantages, surface preparation requirements, and specific applications to titanium alloys, highlighting the formation mechanisms and morphological development during nitriding treatments.
Introduction to Salt Bath Nitriding Technology
Nitriding represents one of the most popular case hardening techniques in modern metallurgy, renowned for delivering exceptional material properties at relatively low process temperatures. Salt bath nitriding, commonly referred to as ferritic nitrocarburizing (FNC), stands as one of the most effective methods to achieve superior surface enhancement results.
This thermochemical process maintains its popularity in industrial applications because it delivers increased hardness and wear resistance at component surfaces while cores remain softer and more ductile. Additionally, the treatment provides enhanced corrosion resistance and significantly reduces the risk of distortion due to comparatively lower treatment temperatures.
The Nitriding Process Fundamentals
Salt bath nitriding operates as a thermochemical process where nitrogen and carbon are simultaneously diffused into the material surface. The high concentration of nitrogen chemically combines with iron and other nitride-forming elements, producing an outer layer of epsilon iron nitride (Fe3N). This resulting layer demonstrates remarkable characteristics: it remains thin, hard, and ductile, providing optimal performance for various industrial applications.
The process temperature requirements remain significantly lower than many alternative hardening techniques, which contributes to reduced thermal stress and minimized component distortion during treatment. This temperature advantage makes salt bath nitriding particularly attractive for precision components where dimensional stability is critical.
Surface Preparation and Process Requirements
Proper surface preparation forms the foundation of successful nitriding operations. Components must undergo thorough cleaning and degreasing procedures before entering the nitriding environment. Any surface contamination from grinding particles, oil, or metal chips will result in uneven formation of the nitrided layer, potentially causing cracks in the coating that lead to flaking and subsequent corrosion.
Following the cleaning phase, parts are systematically dried and preheated before transfer to the actual nitriding environment. The various nitriding processes can be differentiated primarily by their nitrogen source and energy supply methods. Salt bath, gas, and plasma nitriding techniques each offer distinct advantages regarding investment cost, process time, environmental impact, safety considerations, and final quality outcomes.
The properties of the resulting nitrided or nitrocarburized surface remain largely independent of the specific production process selected. However, the required case depth is determined by the intended application of the nitrided component and can be precisely regulated through careful control of nitriding temperature and processing time.
Nitriding Applications for Titanium Alloys
The competitiveness of titanium alloys stems from their exceptional strength-to-weight ratio combined with outstanding heat and corrosion resistance properties. However, these materials face significant limitations including low surface hardness, poor wear resistance, and inadequate high-temperature oxidation resistance. Nitriding treatments specifically target these disadvantages to improve the tribological characteristics of titanium components.
Gas Nitriding of Titanium
In principle, all major nitriding techniques prove applicable to titanium materials. Gas nitriding, while effective, presents certain disadvantages including high temperature requirements ranging from 650-1000°C, extended processing times up to 100 hours, and reported fatigue life reduction in treated components. For Ti-6Al-4V alloy specifically, typical compound layers of 2-15 µm thickness form during treatment, achieving surface hardness values between 500 and 1800 HV.
Plasma Nitriding Advantages
Plasma nitriding of titanium alloys operates at more moderate temperatures of 400-950°C with substantially shorter processing times ranging from 0.5 to 32 hours. This technique generates compound layers with approximately 50 μm thickness. Importantly, any reduction in fatigue strength can be eliminated by implementing lower nitriding temperatures during the process.
Advanced Nitriding Techniques
Ion beam nitriding utilizes nitrogen at temperatures of 500-900°C for processing periods up to 20 hours, producing 5-8 μm thick compound layers with microhardness values of 800-1200 HV on Ti-6Al-4V alloy. Laser nitriding also proves applicable to titanium materials, although the surface case demonstrates a tendency toward cracking.
Recent developments include diode laser gas nitriding techniques applied to Ti6Al4V alloy, commonly used for rotors and blades in power generation engines. The laser surface melting of substrate surfaces in nitrogen and argon mixtures achieves surface hardness increases up to 1300 HV0.2, although outcomes remain dependent on specific process parameters.
Formation Mechanisms in Titanium Nitriding
The formation of nitrided layers on titanium involves several complex reactions occurring at the gas/metal interface and within the metal structure itself. At nitriding temperatures below the titanium polymorphic transformation, the α-Ti phase exists as the dominant structure.
Initially, nitrogen absorbed at the surface diffuses inward through the titanium, forming an interstitial solution of nitrogen in the hexagonal close-packed titanium phase α-Ti(N) and establishing a nitrogen concentration gradient. After the solubility limit is exceeded, the Ti2N phase begins formation. During continued nitrogen concentration increases at the gas/metal interface, TiN forms according to the following progression:
α - Ti → α – TiN → Ti2N → TiN
Following slow cooling procedures, precipitation in the diffusion zone becomes possible.
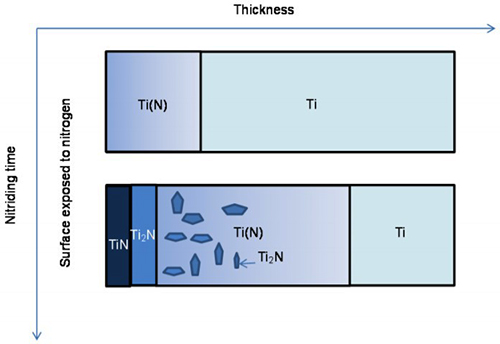
Figure 1: Schematics of the morphology development during nitriding of titanium
Conclusion
Liquid salt bath nitriding continues to demonstrate its value as a versatile and effective case hardening technique across diverse industrial applications. The process delivers exceptional improvements in surface hardness, wear resistance, and corrosion protection while maintaining the beneficial properties of base materials. For titanium alloys specifically, various nitriding approaches offer solutions to inherent surface property limitations, enabling expanded applications in demanding environments.
The selection of appropriate nitriding parameters, including temperature, time, and specific technique, requires careful consideration of component requirements, material characteristics, and desired performance outcomes. As technology continues advancing, nitriding processes will likely evolve to provide even greater precision and efficiency in surface enhancement applications.
チタン合金の正確な特性を今すぐチェック!
Total Materia Horizon には、数千種類のチタン合金の組成、温度別の機械的・物理的特性、非線形特性などが収録されています。
Total Materia Horizonの無料テストアカウントを開設して、120カ国以上、50万人を超えるユーザーのコミュニティに参加しましょう!