Hydrogen in Steels
Abstract
This comprehensive study examines the critical role of hydrogen control in steel manufacturing and its significant impact on product quality. Hydrogen contamination, even at concentrations of a few parts per million, can cause severe defects including flaking, embrittlement, and reduced ductility. The article explores thermodynamic principles governing hydrogen behavior in steel, analyzes various contamination sources during production, and presents experimental data on hydrogen pickup through calcium hydroxide and coke additions. Practical recommendations for hydrogen content control in modern steelmaking processes are provided, emphasizing the importance of proper material selection and process optimization for quality steel production.
Understanding Hydrogen's Impact on Steel Quality
The presence of hydrogen in steel manufacturing presents persistent challenges that significantly affect both production processes and final product performance. When hydrogen content exceeds the solubility limit in solid iron, the rejection process during solidification initiates a cascade of detrimental effects.
The primary concerns include:
- Formation of structural defects (pinholes and internal porosity)
- Development of hairline cracks, known as flakes
- Occurrence of hydrogen embrittlement
- Reduction in tensile ductility
- Surface blistering in finished products
These issues become particularly critical in larger steel products such as heavy section castings, ingots, blooms, and slabs, where hydrogen removal is more challenging due to:
- Extended diffusion distances
- Complex cooling patterns
- Greater internal stress development
- Limited effectiveness of conventional hydrogen removal methods
The severity of these effects varies with product dimensions and processing conditions, making hydrogen control essential throughout the entire manufacturing process. Understanding these fundamental impacts guides the development of effective control strategies and quality assurance measures.
Thermodynamic Principles and Hydrogen Solubility
The behavior of hydrogen in steel follows specific thermodynamic principles, primarily governed by Sievert's law. This fundamental relationship describes how diatomic hydrogen gas dissolves into its atomic form within the metal matrix. The process can be expressed through the following essential equation:
½H2(G) = [H] (dissol. in metal) (1)
With its corresponding equilibrium constant:
K= [ppm H] / (pH2)½ (2)
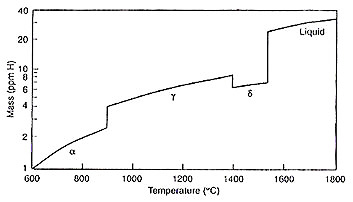
Figure 1: Solubility of Hydrogen in pure iron or low-alloy steel at 1 atm pressure of H2
The temperature dependence of the equilibrium constant varies across different iron phases, as described by the following relationships (where T is in Kelvin):
For α, δ(bcc) iron:
log K α,δ= -1418/T+ 1.628 (3)
For γ(fcc) iron:
log K γ = -1182/T+ 1.628 (4)
For liquid iron (I):
log K I = -1900/T+ 2.423 (5)
Hydrogen Pressure Development During Cooling
During the rapid cooling of heavy-section steel castings, such as thick slabs or blooms, hydrogen diffusion becomes severely limited. This limitation, combined with decreasing hydrogen solubility at lower temperatures, results in significant hydrogen pressure buildup within the steel matrix.
Table 1. Build-up of H2 pressure in the steel matrix during rapid cooling
| Temperature, °C | H2 pressure, atm | |||
| 2 ppm H | 4 ppm H | 8 ppm H | ||
| γ | 1400 | 0.058 | 0.23 | 0.90 |
| γ | 1100 | 0.12 | 0.48 | 1.90 |
| γ | 900 | 0.23 | 0.92 | 3.70 |
| α | 900 | 0.58 | 2.33 | 9.30 |
| α | 700 | 1.83 | 7.30 | 29.2 |
| α | 500 | 10.4 | 41.6 | 166.5 |
The pressure development varies significantly based on:
- Initial hydrogen content (2, 4, or 8 ppm)
- Temperature range
- Phase transformation points
Key observations from pressure development data:
- Pressure increases dramatically as temperature decreases
- Phase transformation from γ to α results in substantial pressure jumps
- At 500°C, pressure can reach as high as 166.5 atm for 8 ppm hydrogen content
This pressure buildup phenomenon explains many of the observed defects in larger steel products and emphasizes the critical importance of controlled cooling rates and effective hydrogen management strategies.
Sources of Hydrogen Contamination and Pickup Mechanisms
The introduction of hydrogen during steel manufacturing occurs through multiple pathways, primarily during melting, ladle processing, and casting operations. Primary contamination sources include:
Water-associated contamination:
- Moisture in slag-making materials
- Hydrated raw materials
- Atmospheric humidity exposure
Process-specific sources:
- Alloy additions containing hydrogen impurities
- Carburizer contamination
- Natural gas (CH4) used as tuyere coolant in bottom-blown converters (Q-BOP)
A significant source of hydrogen contamination occurs through lime (CaO) additions, particularly during ladle metallurgy operations. This process follows two key chemical reactions:
Ca(OH)2 (s) = CaO(s) + H2O(g) (6)
H2O(g) = 2[H] + [O] (7)
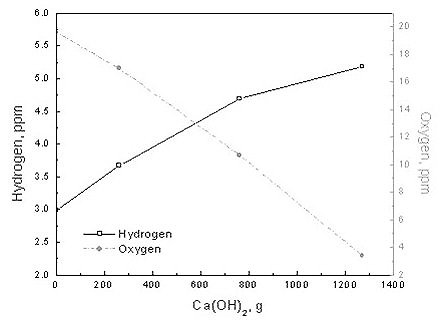
Figure 2: Hydrogen pickup due to Ca(OH)2 addition on the top of the slag
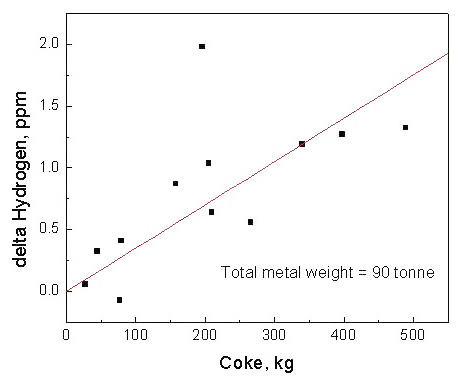
Figure 3: Change in the hydrogen content of the liquid metal as a function of coke addition for 90 tone heat
Control Strategies and Best Practices
The control of hydrogen content in steel remains a critical task for steelmakers, particularly during BOF and EAF steelmaking operations. Based on the experimental trials and industrial experience, effective hydrogen control requires a systematic approach focused on several key aspects of the production process.
The primary control measures must focus on material inputs and process parameters. This includes using raw materials with low moisture or hydrogen content and avoiding the late addition of lime during the smelting process. Operational controls should emphasize the minimization of carry-over slag and implementation of efficient degassing procedures using deep vacuum and intense purging.
Additional control measures include:
- Recarburization using low hydrogen pet coke
- Careful control of ladle slag basicity
- Proper timing of material additions
The experimental results demonstrate that hydrogen pickup increases with both lime (Ca(OH)2) and coke additions. However, the steel's capacity to absorb hydrogen decreases as the hydrogen content approaches equilibrium value. An important finding shows that the capacity to absorb hydrogen increases as the steel is deoxidized.
Accédez en quelques instants aux compositions précises des matériaux !
Total Materia Horizon contient les compositions chimiques de centaines de milliers de matériaux, ainsi que leurs propriétés mécaniques et physiques, et bien plus.

Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.