Corrosion protection of Steel: Part One
Abstract
This comprehensive article explores the critical aspects of steel corrosion protection, focusing on both the mechanisms of corrosion and various protective methods. The text examines three primary protection strategies: passive barrier protection, active protection, and sacrificial protection, with particular emphasis on modern coating technologies. Detailed analysis is provided on electrocoating (E-coating), metallic coatings, organic coatings, and powder coating processes, offering practical insights into their applications and effectiveness. The article addresses how these methods combat the electrochemical reaction requiring water, oxygen, and ions that causes steel corrosion, providing essential knowledge for industry professionals and engineers working in corrosion prevention.
Understanding the Fundamentals of Steel Corrosion
Corrosion is a complex electrochemical phenomenon that causes significant deterioration in metals through oxidization. This process results in millions of dollars in losses throughout the metal industry annually. The mechanism occurs when two metals with different potentials are coupled together in a conductive electrolyte, initiating current flow and subsequent corrosion. The corrosion process primarily occurs at points where current exits the metal surface.
The corrosion of steel specifically requires three key elements: water (H2O), oxygen (O2), and ions such as chloride ions (Cl¯), all naturally present in the atmosphere. Coastal areas are particularly susceptible to corrosion due to higher concentrations of atmospheric chloride ions. This electrochemical reaction initiates when atmospheric oxygen oxidizes iron in the presence of water.
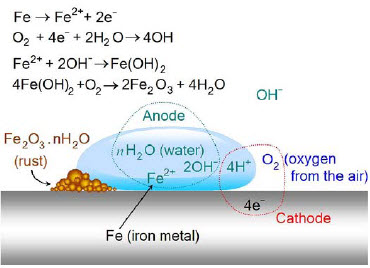
Figure 1: Schematic representation of the corrosion process
Understanding Corrosion Protection Methods for Steel
1. Passive Barrier Protection Systems
This fundamental protection method involves creating a protective coating system that forms an impermeable barrier between the steel and corrosive elements (oxygen, water, and ions). The effectiveness of this method directly correlates with the coating's impermeability to water. High-performance coatings such as two-pack epoxy systems and chlorinated rubbers, when applied at appropriate film thickness, provide superior corrosion protection through this passive barrier mechanism.
2. Active Protection Mechanisms
Active corrosion protection employs a more sophisticated approach, utilizing reactive chemical compounds applied directly to the steel surface. These compounds work by disrupting the natural formation of anodes on the steel surface. A prime example is the use of zinc phosphate (Zn3(PO4)2), an inorganic zinc inhibitive pigment. When exposed to water, it undergoes hydrolysis, producing zinc ions (Zn2+) and phosphate ions (PO43-). The phosphate ions function as anodic inhibitors by phosphating the steel and inducing passivity, while zinc ions serve as cathodic inhibitors.
3. Sacrificial Protection Technology
Also known as cathodic or galvanic protection, this method leverages the natural electrochemical behavior between dissimilar metals. Zinc stands out as the most widely utilized protective metal for steel, offering protection through preferential oxidation. The effectiveness of zinc stems from its dual advantages: it not only corrodes preferentially to steel but also typically corrodes at a slower rate. However, it's important to note that this corrosion rate accelerates in chloride-rich environments, such as coastal areas.
Advanced Coating Technologies in Steel Protection
Electrocoating (E-coat) Technology
Electrocoating represents a significant advancement in corrosion protection technology. This sophisticated process, developed in the 1930s and refined through extensive research in Europe during the 1960s, involves depositing electrically charged particles from a water suspension onto conductive materials. Now widely adopted across North America, E-coating has become essential for protecting various metal components, from simple stampings to complex automotive bodies.
The E-coating process requires:
- A specialized coating tank for part immersion
- Precise temperature control systems
- Advanced filtering equipment
- Circulation maintenance systems
The process can be either anodic or cathodic, with cathodic systems being preferred due to their superior corrosion resistance and reduced surface preparation requirements. During the process, coating materials (binder, pigment, and additives) receive an electrical charge and migrate through water under an electric field. Upon reaching the part surface, these materials undergo charge neutralization by electrochemically generated OH¯, ions in cathodic processes, forming a uniform coating layer typically ranging from 10 to 30 micrometers.
Metallic Coating Applications
Metallic coatings serve dual purposes in protecting both ferrous and non-ferrous substrates: corrosion inhibition and aesthetic enhancement. The selection of specific coating materials depends on several factors:
- The severity of the corrosive environment
- Expected wear and abrasion exposure
- Visibility requirements of the component in service
Four primary application methods have been developed for metallic coatings:
- Electroplating: Utilizes electrical potential to deposit coatings through an electrolyte solution
- Mechanical Plating: Involves cold welding of metal powder through tumbling processes
- Electroless Plating: Employs chemical reactions for metal deposition without electrical current
- Hot Dipping: Involves immersion in molten coating metal baths
Advanced Surface Protection Methods
Organic Coating Systems
Organic coatings represent one of the most cost-effective approaches to corrosion protection. These systems function by creating an electrochemical barrier between the corrosive environment and the metal substrate. The protective mechanism works by preventing the transfer of electrochemical charge from corrosive solutions to the underlying metal, while simultaneously creating a physical barrier against environmental contaminants. This results in a controllable and uniform protective layer that effectively shields the metal surface.
The autodeposition process in organic coating applications demonstrates unique characteristics, with film thickness development following a time and temperature-dependent pattern. The process begins with an initial rapid deposition phase, gradually slowing as the film matures. While deposition continues throughout the immersion period, the rate progressively declines as the coating builds up. Typical film thicknesses are precisely controlled between 15 to 25 micrometers (0.6 to 0.8 mils).
A significant advantage of this method is its versatility in coating complex geometries, including tubular structures, assembled components, and intricate design features. The process requires no phosphate stage and can be cured at relatively low temperatures, making it both efficient and economical.
Powder Coating Technologies
Powder coating represents a modern approach to surface protection, offering distinct advantages over traditional liquid coatings. The process involves applying dry powder to clean surfaces, followed by heat fusion to create a smooth, continuous film. The technology has evolved to accommodate a wide range of chemical types, including acrylic compounds, vinyl formulations, epoxy-based materials, nylon compositions, polyester variants, and urethane formulations.
The application of powder coatings can be achieved through four primary methods:
- Fluidized bed process
- Electrostatic bed process
- Electrostatic spray process (most commonly used)
- Plasma spray process
The electrostatic spray process has become the industry standard due to its efficiency and reliability. In this process, electrically conductive and grounded objects are sprayed with charged, non-conducting powder particles. These particles are attracted to the substrate through electrostatic forces and adhere to the surface. Subsequent heating in an oven fuses the particles into a smooth, continuous film. The resulting coating typically ranges from 25 to 125 micrometers in thickness, though achieving very thin film thicknesses can be challenging.
One of the most significant advantages of powder coating technology is its material efficiency. Modern systems incorporate booths and collection systems that capture overspray, allowing for material reuse and reducing waste. This feature, combined with the durability and quality of the finished coating, makes powder coating an increasingly popular choice for industrial applications.
En savoir plus
Accédez immédiatement à des données précises en corrosion !
Total Materia Horizon contient le comportement en corrosion et des informations sur les propriétés pour des centaines de milliers de matériaux, dans plus de 2000 milieux corrosifs.
Profitez d’un compte d’évaluation GRATUIT sur Total Materia Horizon et rejoignez notre communauté qui compte plus de 500.000 utilisateurs dans plus de 120 pays.