Continuous Electroplating Process for Steel Sheet Products
Abstract
The continuous electroplating process for steel sheet products applies the fundamental principles of conventional decorative electroplating but operates at high speeds through multiple plating cells. This process builds coating thickness incrementally as steel strip passes through individual cells at speeds of 150-200 meters per minute. Modern continuous electroplating lines primarily produce zinc, zinc/nickel, and zinc/iron alloy coatings for automotive and industrial applications. The process requires substantial electrical power consumption and precise solution control to maintain coating quality. Electrogalvanized coatings offer excellent surface finish and corrosion protection equivalent to hot-dip galvanized products at identical coating masses, making them particularly suitable for exposed automotive body panels when combined with additional protective treatments.
Introduction
The development of modern continuous sheet galvanizing lines has led to the disappearance of most manual mills for galvanizing cut sheets. However, some machines still galvanize cut-to-length sheets using chemical pretreatment sequences similar to those for wire or tube galvanizing.
At the beginning of the line, operators weld the end of one coil to the start of the next coil. Two basic methods exist for continuously galvanizing sheet, differing in how the strip is cleaned before galvanizing: chemically or through thermal treatments. Coils of annealed cold-reduced sheet may be fed directly to the galvanizing line, or alternatively, coiled sheet undergoes continuous heat treatment in the pretreatment line. After leaving the galvanizing bath, where the strip remains for only a few seconds, the surface is wiped to remove excess zinc and may receive further treatment to alter surface appearance, composition, smoothness, or mechanical properties.
Steel Sheet Electroplating Process Fundamentals
The steel sheet electroplating process utilizes the same basic principle as conventional decorative finish electroplating. However, the steel sheet process differs significantly in that the electroplated coating is applied by passing the strip at high speeds through a series of plating cells, building coating thickness incrementally as the strip passes through each individual cell. This continuous process for electroplating steel strip requires specialized equipment to transport the strip at high speeds (150 to 200 meters per minute and higher) through a series of individual plating cells, presenting more complexity than initially apparent.
Electroplating Cell Design and Operation
The simplest electroplating cell configuration uses a zinc sulfate plating solution bath, as illustrated in the accompanying diagram.
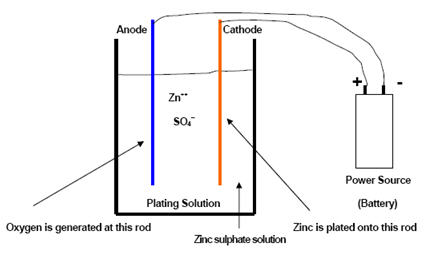
Figure 1: The common schema of the electroplating cell
This simple plating cell demonstrates the fundamental actions during the plating process. At the cathode (steel, for example), zinc ions dissolved in the zinc sulfate solution combine with two electrons and form elemental zinc, which deposits onto the cathode surface. At the anode, water converts to oxygen and hydrogen ions to maintain electrical balance. The oxygen forms a gas, and nothing deposits on the anode surface. The plating solution carries the current between the cathode and anode.
Continuous Steel Sheet Plating Systems
Multiple anode arrangement types exist in modern electroplating systems. Some configurations are horizontal, others are vertical, and one process utilizes a radial cell where the strip passes around large-diameter rolls inside each plating cell. The anodes feature a radial design matching the diameter of the large rolls submerged in the plating solution. Each anode arrangement and design offers distinct advantages and disadvantages, explaining why different manufacturers employ different methods. Each system requires precise control of the anode-to-strip spacing to achieve efficient plating while avoiding arc spots and other coating defects.

Figure 2: Modern Continuous Electroplating Line
Maintaining the large volume of plating solution contained in all cells represents a specialized science. Whether the electrogalvanizing plating solution uses zinc sulfate or zinc chloride chemistry, maintaining proper ranges of zinc ion concentration and solution pH constitutes critical control features. Beyond plating zinc, some manufacturers possess the capability to deposit alloy coatings, requiring at minimum one additional level of plating solution control. For example, producing a zinc/nickel alloy coating demands close control of both dissolved zinc and nickel concentrations in the solution. Solution control must be accomplished dynamically since these lines operate continuously.
Power Requirements for Electroplating Operations
The electroplating process demands substantial electric power to deposit metallic coatings. Total power requirements directly correlate with the coating thickness needed to meet customer specifications. For example, the power required to deposit a zinc coating mass of 80 g/m² is approximately twice that required to deposit a coating of 40 g/m². A typical line capable of processing 70 to 120 tons per hour with a coating mass of 50 g/m² will consume hundreds of thousands of amperes during one hour of processing time. Power costs clearly represent a major cost component for facilities processing large quantities of electroplated sheet products.
Electroplated Steel Sheet Product Types
The most common electroplated coating for steel sheet products is zinc. Electrogalvanized zinc coatings are utilized by numerous automotive companies for exposed car-body panels, where typical coating mass ranges from approximately 50 to 80 g/m² per side. These coatings are considerably thicker than electrogalvanized coatings typically used for non-automotive applications, so lines built for automotive applications usually incorporate numerous plating cells. Additionally, each automotive customer maintains specific coated-product specifications.
Another attribute associated with electrogalvanized coatings for automotive applications is the excellent surface finish attainable through the electroplating process. Twenty-five years ago, when automotive companies began using substantial amounts of galvanized sheet for exposed body panels to improve corrosion protection, electrogalvanized products were among the few coated sheet products meeting demanding surface quality requirements. Hot-dip galvanized products were, and continue to be, used for unexposed body parts. As hot-dip product surfaces improve, they continue replacing electrogalvanized sheet for exposed automotive body panels.
Other zinc electroplating lines have been constructed over the years to produce thinner coatings. Typically, sheet produced on these lines has coating mass less than 25 g/m². Applications for this product are often indoor environments where conditions are not highly corrosive. Many applications involve painted products. These coating lines often possess the capability to apply paint pretreatment, allowing customers to paint directly without additional in-house treating.
A second type of electroplated coated-steel sheet manufactured today features a zinc/nickel alloy coating. Typically, nickel content ranges from 10 to 16 percent with the balance being zinc. The unique feature of this process is that zinc and nickel ions are co-deposited to create a true alloy coating rather than alternating layers.
Applications for this product have been limited primarily to select automotive companies. These companies have developed in-house product design and manufacturing processes to leverage the unique characteristics of zinc/nickel coatings. For these automotive applications, the metallic coating is often covered with a special corrosion-resistant thin organic coating applied over the zinc/nickel layer. The zinc/nickel alloy coating is covered by ASTM Specification A 918.
A third type of electroplated coating is zinc/iron alloy coating. The attributes of this specialized coating somewhat resemble those of hot-dip galvannealed products. Like zinc/nickel alloy, zinc/iron coating is co-deposited as an alloy coating with iron uniformly deposited throughout the coating thickness. Also like zinc/nickel coating, zinc/iron coating is used predominantly by the automotive industry. The attributes of electroplated zinc/iron include relatively easy welding and painting when proper electro-priming equipment is available to automotive manufacturers. Additionally, the coating is very hard, making it less susceptible to scratching during stamping and handling. This represents an important feature since zinc/iron alloy coated-sheet products are used almost exclusively for exposed car-body panels.
Corrosion Resistance of Electroplated Coatings
Regarding the corrosion behavior of electrogalvanized versus hot-dip galvanized coatings, it is important to note that performance is essentially equivalent for identical coating masses. A coating mass of 100 g/m² will provide essentially the same corrosion protection whether it is hot-dip galvanized or electrogalvanized.
Automotive companies can successfully use coating masses in the 50 to 80 g/m² range because they apply additional treatments over the metallic coating, including zinc phosphate coating, electro-deposited organic-based coating, primer, and multiple-layer finishing paint coatings. Clearly, the corrosion resistance needed to protect car body panels for over 10 years exceeds that afforded by metallic coating alone. Application of these coatings over the electroplated metallic layer results in a synergistic system whose corrosion resistance exceeds the sum of its individual components.
¡Acceda Ahora a las Propiedades Precisas de los Aceros Estructurales!
Total Materia Horizon contiene información sobre las propiedades de más de 150.000 aceros estructurales: composición, propiedades mecánicas y físicas, propiedades no lineales y mucho más.
Obtenga una cuenta de prueba GRATUITA de Total Materia Horizon y únase a nuestra comunidad que traspasa los 500.000 usuarios provenientes de más de 120 países.